《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines

Amines Amines are derivatives of ammonia with one or more alkyl groups bonded to the nitrogen. Amines can be classified as primary,secondary or tertiary,meaning one,two and three alkyl groups bonded to the nitrogen respectively E.g. CH3 Primary -NH2 H3C-C-NH2 Amines CH3 CH3 Secondary N-H Amines Tertiary Amines Quaternary ammonium salts have four alkyl groups bonded to the nitrogen,and the nitrogen bears a full positive charge E.g. CH2CH3 H3CH2C-N-CH2CHs r N-CH2CH2CH3 CH2CHa Br Ch19 Amines(landscape) Page I
Ch19 Amines(landscape) Page 1 Amines Amines are derivatives of ammonia with one or more alkyl groups bonded to the nitrogen. Amines can be classified as primary, secondary or tertiary, meaning one, two and three alkyl groups bonded to the nitrogen respectively. E.g. Quaternary ammonium salts have four alkyl groups bonded to the nitrogen, and the nitrogen bears a full positive charge. E.g. NH2 H3C C CH3 CH3 NH2 Primary Amines Secondary Amines N: H CH3 N-H Tertiary Amines N: CH3 CH3 N H3CH2C N CH2CH3 CH2CH3 CH2CH3 + I - N CH2CH2CH3 + Br -

Amines are a very common functional group in organic chemistry,and especially so for naturally occurring compounds. E.g. CH3 O OCH 0 0 Ph H CH3 cocaine nicotine in coca leaves in tobacco CH-CH2NH -CH3 CHO OCH3 OCH3 OH OH H mescaline morphine in peyote cactus in opium poppies Ch19 Amines(landscape) Page 2
Ch19 Amines(landscape) Page 2 Amines are a very common functional group in organic chemistry, and especially so for naturally occurring compounds. E.g

Nomenclature The IUPAC nomenclature is analogous to that for alcohols,except the -e ending is replaced with-amine. Other substituents on the carbon chain are given numbers,and the prefix N-is used for each substituent on nitrogen E.g NH2 CH3 NH2 CHCH2CHCH: CHCHCH2CH2 old IUPAC names in blue:2-butanamine 3-methyl-1-butanamine new IUPAC names in green:butan-2-amine 3-methylbutan-I-amine 2013 Pearson Education.Inc. NHCH3 CH3 CH3 CHCH2CHCH3 CHCH2CHCHCHCH :N(CH3)2 N-methyl-2-butanamine 2.4.N.N-tetramethyl-3-hexanamine N-methylbutan-2-amine 2.4.N.N-tetramethylhexan-3-amine Ch19 Amines(landscape) Page 3
Ch19 Amines(landscape) Page 3 Nomenclature The IUPAC nomenclature is analogous to that for alcohols, except the -e ending is replaced with -amine. Other substituents on the carbon chain are given numbers, and the prefix N- is used for each substituent on nitrogen. E.g

Aromatic amines are called by their historical/trivial names,with phenylamine being called aniline H H N(CH2CH3)2 CH2CH3 CH3 aniline 2-ethylaniline N.N-diethylaniline 4-methylaniline or o-ethylaniline or p-toluidine Other nitrogen heterocycles have ring system names that need to be learnt also.(The N is normally considered to be numbered 1). CH3 pyrrole pyrrolidine 1-methylpyrrolidine imidazole ndo (N-methylpyrrolidine) CH3 pyridine 2-methylpyridine piperidine pyrimidine Ch19 Amines(landscape) Page 4
Ch19 Amines(landscape) Page 4 Aromatic amines are called by their historical/trivial names, with phenylamine being called aniline. Other nitrogen heterocycles have ring system names that need to be learnt also. (The N is normally considered to be numbered 1)

Structures of Amines Previously we have seen that ammonia(NH3)has a slightly distorted tetrahedral shape,due the compression of the ideal 109.5 angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups,and trimethylamine has bond angles closer to the perfect sp arrangement than ammonia. NgH N-CH3 H107 CH3 108 ammonia trimethylamine electrostatic potential map of trimethylamine Since an amine has three substituents and a lone pair,the question of chirality arises. If an amine has three different substituents(and its lone pair)can we resolve the amine into enantiomers? Ch19 Amines(landscape) Page 5
Ch19 Amines(landscape) Page 5 Structures of Amines Previously we have seen that ammonia (NH3) has a slightly distorted tetrahedral shape, due the compression of the ideal 109.5° angle by lone pair-bond pair repulsion. This effect is less pronounced with alkyl groups, and trimethylamine has bond angles closer to the perfect sp3 arrangement than ammonia. Since an amine has three substituents and a lone pair, the question of chirality arises. If an amine has three different substituents (and its lone pair) can we resolve the amine into enantiomers?

In most cases,this is not possible since the enantiomers can interconvert through a low energy pathway The interconversion takes place through a nitrogen inversion,where the lone pair moves from one face of the molecule to the other,and back sporbital p orbital CH3 CH2CH3 N H CH3 H CH3 CH2CH3 sporbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine The lone pair starts off in an sp'orbital,but in the transition state of the inversion,the nitrogen can rehybridize to sp,with the lone pair in a p orbital. This is not a high energy situation,and only requires 6kcal of energy to achieve this TS(therefore easy at room temperature). At the TS,the inversion can occur or return back to the original enantiomer-single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention,lone pairs have the lowest priority). Ch19 Amines(landscape) Page 6
Ch19 Amines(landscape) Page 6 In most cases, this is not possible since the enantiomers can interconvert through a low energy pathway. The interconversion takes place through a nitrogen inversion, where the lone pair moves from one face of the molecule to the other, and back. The lone pair starts off in an sp3 orbital, but in the transition state of the inversion, the nitrogen can rehybridize to sp 2 , with the lone pair in a p orbital. This is not a high energy situation, and only requires 6kcal of energy to achieve this TS (therefore easy at room temperature). At the TS, the inversion can occur or return back to the original enantiomer - single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention, lone pairs have the lowest priority)
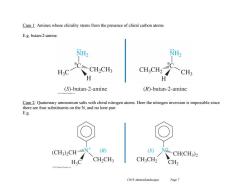
Case 1:Amines whose chirality stems from the presence of chiral carbon atoms. E.g.butan-2-amine. CH.CH CH3 H (S)-butan-2-amine (R)-butan-2-amine 020 Case 2:Quaternary ammonium salts with chiral nitrogen atoms.Here the nitrogen inversion is impossible since there are four substituents on the N,and no lone pair. E.g. (CH3)2CHN (R) (S) NiCH(CH3)2 CH2CH3 CHCH2 CH3 Ch19 Amines(landscape) Page 7
Ch19 Amines(landscape) Page 7 Case 1: Amines whose chirality stems from the presence of chiral carbon atoms. E.g. butan-2-amine. Case 2: Quaternary ammonium salts with chiral nitrogen atoms. Here the nitrogen inversion is impossible since there are four substituents on the N, and no lone pair. E.g

Case 3:Certain amines cannot attain the sp'hybridization required for nitrogen inversion. Examples of this include nitrogen atoms in small rings(aziridines). H3 CH; H30 CH; CH3 H3C (R)-1,2,2-trimethylaziridine (S)-1,2,2-trimethylaziridine The required bond angle of 120 is unobtainable in the strained system,and so the TS required for nitrogen inversion is of too high energy,and thus chiral aziridines can be resolved into enantiomers. Ch19 Amines(landscape) Page 8
Ch19 Amines(landscape) Page 8 Case 3: Certain amines cannot attain the sp2 hybridization required for nitrogen inversion. Examples of this include nitrogen atoms in small rings (aziridines). The required bond angle of 120° is unobtainable in the strained system, and so the TS required for nitrogen inversion is of too high energy, and thus chiral aziridines can be resolved into enantiomers

Basicity of Amines The nitrogen atom of amines has a lone pair of electrons,and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile H H R一NCH3F H nucleophile electrophile new N-C bond formed Amine as a base: H R一Nt- H X H base proton acid protonated Amines are basic,and therefore their aqueous solutions are basic (pH>7),and recall that base strength is talked of it terms of base-dissociation constant(Kp). Ch19 Amines(landscape) Page 9
Ch19 Amines(landscape) Page 9 Basicity of Amines The nitrogen atom of amines has a lone pair of electrons, and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile: Amine as a base: Amines are basic, and therefore their aqueous solutions are basic (pH>7), and recall that base strength is talked of it terms of base-dissociation constant (Kb)

H R-N: H-0-HR-H+0附 + H K,= [RNH3][OH] [RNH2] pKb=-l0g10Kb The values of K for most amines are small (10),but still basic. Since amine basicity values span many orders of magnitude,discussion of pKo values is more common. Remember pKb=-logio Kb And that KaK=Kw=10-14 Ch19 Amines(landscape) Page 10
Ch19 Amines(landscape) Page 10 The values of Kb for most amines are small (10-3 ), but still basic. Since amine basicity values span many orders of magnitude, discussion of pKb values is more common. Remember pKb = -log10 Kb And that KaKb=Kw = 10-14
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 12 Infrared Spectroscopy and Mass Spectrometry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 11 Reactions of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 10 Synthesis and Structure of Alcohols.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 09 Alkynes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 08 Reactions of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 07 Structure and Synthesis of Alkenes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 06 Alkyl Halides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 05 Stereochemistry.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 04 Rates & Kinetics.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 03 Alkanes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 02 Structure and Properties.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 01 Introduction.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 27 Synthetic Polymers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry with Biological Applications, 3th Edition, John McMurry, 2016)Chapter 26 Orbitals and Organic Chemistry - Pericyclic Reactions.ppt
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
