《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Chemistry of Aromatic Compounds

Chemistry of Aromatic Compounds Electrophilic Aromatic Substitution Directing Effects Side-chain Reactions Synthesis Nucleophilic Aromatic Substitution
Chemistry of Aromatic Compounds Electrophilic Aromatic Substitution Directing Effects Side-chain Reactions Synthesis Nucleophilic Aromatic Substitution
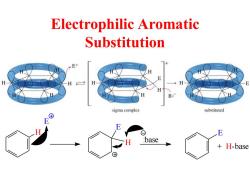
Electrophilic Aromatic Substitution E+ H H H B sigma complex substituted E :base + H-base ⑧
Electrophilic Aromatic Substitution H E E H E + H-base :base
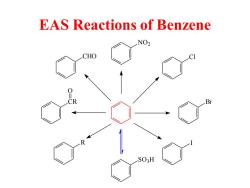
EAS Reactions of Benzene NO2 CHO SO3H
EAS Reactions of Benzene Br NO2 SO3H CR O Cl R I CHO
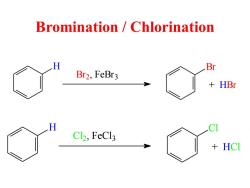
Bromination /Chlorination H Br Br2,FeBr3 HBr C12,FeCl3 HCI
Bromination / Chlorination H Br Br2, FeBr3 + HBr H Cl C l2, FeCl3 + HC l
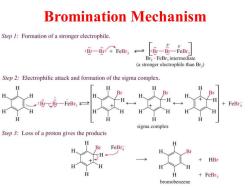
Bromination Mechanism Step 1:Formation of a stronger electrophile. :B-Br:FeBr :Br-Br-FeBr Br2FeBr,intermediate (a stronger electrophile than Br,) Step 2:Electrophilic attack and formation of the sigma complex. H B H :Br-CBr-FeBr H +FeBra H H H 人 sigma complex Step 3:Loss of a proton gives the products H FeBra Br H H H +FeBr3 bromobenzene
Bromination Mechanism
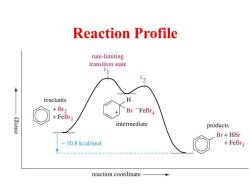
Reaction Profile rate-limiting transition state reactants H +Br2 Br-FeBr4 K3.Ioua +FeBr3 intermediate products Br+HBr 10.8 kcal/mol +FeBr3 reaction coordinate
Reaction Profile
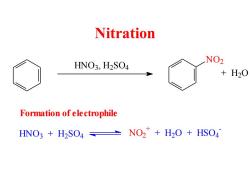
Nitration HNO3,H2SO4 NO2 +H20 Formation of ele ctrophile HNO3 H2SO4 NO2+H2O+HSO4
Nitration HNO3 , H2 SO4 NO2 + H2 O HNO3 + H2 SO4 N O2 + + H2 O + HSO4 - Formation of electrophile
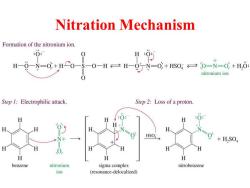
Nitration Mechanism Formation of the nitronium ion. H:0 H--N=+H0-S-0-H=H-95N=+s0=0=N=0+H,6: nitronium ion Step 1:Electrophilic attack. Step 2:Loss of a proton. H H :0: H H H NH HSO> H,SO H H 0 H H H H benzene nitronium sigma complex nitrobenzene ion (resonance-delocalized)
Nitration Mechanism
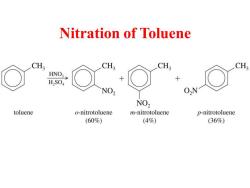
Nitration of Toluene CH; CH; CH; CH3 HNO3 H2SO NO2 O2N NO2 toluene o-nitrotoluene m-nitrotoluene p-nitrotoluene (60%) (4%) (36%)
Nitration of Toluene
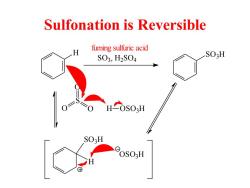
Sulfonation is Reversible fuming sulfuric acid SO3,H2SO4 SO:H SO:H OSO3H
Sulfonation is Reversible H SO3, H2 SO4 SO3H fuming sulfuric acid S O O O H OSO3H SO3H H OSO3H
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Stereochemistry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acids Nitriles.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Carboxylic Acid Derivatives.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Benzene and Aromaticity(2011).ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkynes McMurry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkyl Halides from Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alkenes Overview.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Alcohols-structure and synthesis 2.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Structure and Bonding of Organic Molecules.pptx
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)CHM 201 Introduction and Review - Structure and Bonding.pptx
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 21 Carboxylic acid Derivatives.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 20 Carboxylic Acids.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 19 Amines.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 18 Ketones and Aldehydes.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 17 Reactions of Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 16 Aromatic Compounds.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 15 Conjugated Systems.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 14 Ethers and Epoxides.pdf
- 《有机化学》课程教学课件(Organic Chemistry, Alex Jonathan Roche lecture notes Rutgers The State University NJ, wade 8th)Chapter 13 Nuclear Magnetic Resonance(NMR)Spectroscopy.pdf
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conformational Analysis.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Conjugated Dienes and U.V. Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Enols and Enolates.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ethers.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Functional Groups.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared(IR)spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Ketones and Aldehydes.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Mass Spectrometry.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Nomenclature of Saturated Hydrocarbons.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Organic Reactions.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Proton NMR Spectroscopy.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Reactions of Alcohols.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Substitution and Elimination.ppt
- 《有机化学》课程PPT教学课件(Organic Chemistry, William A. Price, Ph.D. PPT, La Salle University, L.G.WADE, JR., 8th Edition)Infrared Spectroscopy and Mass Spectrometry.ppt
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 01 Structure And Bonding(主讲:王俊儒).pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 02 Alkanes and cycloalkane.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 03 functional groups.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 04 stereochemistry.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 05 Nucleophiles and Electrophiles.pdf
- 西北农林科技大学:《有机化学 Organic chemistry》课程教学课件(2009)Chapter 06 reactions and mechanisms.pdf
