《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)03 Optical Spectroscopy and Instrumentation

Optical Spectroscopy and Instrumentation (Chapter 7) (IR,visible and UV) pathlength b concentration c Transmittance T=I 10 Percent Transmittance %T= 1×100% Io Absorbance A=-logT =-o80 I 二1og1 Examples: T=1.00(100%T),A=0.00 T=0.10(10%T),A=1.00 T=0.001(0.1%T),A=3.00 CEM 333 page 3.1
Optical Spectroscopy and Instrumentation (Chapter 7) (IR, visible and UV) I0 I pathlength b concentration c Transmittance T = I I0 Percent Transmittance %T = I I0 ´100% Absorbance A = -logT = -log I I 0 = log I0 I Examples: T=1.00 (100 %T), A=0.00 T=0.10 (10 %T), A=1.00 T=0.001 (0.1 %T), A=3.00 CEM 333 page 3.1

Beer's Law: Basis for absorbance spectrophotometry A ccc and A ocb S0Acb·c A=ab·c proportionality constant absorptivity units of L/g.cm If units of concentration are M(mol/L)then use molar absorptivity s A=8.b.c units of L/mol.cm Phenomena used for optical measurements, (1)Absorption(2)Emission (3)Luminescence (Fluorescence, Phosphorescence,Chemiluminescence(4)Scattering In all cases,response is proportional to concentration of analyte CEM 333 page 3.2
Beer's Law: Basis for absorbance spectrophotometry A µ c and A µ b so A µ b ×c A = a × b× c proportionality constant absorptivity - units of L/g·cm If units of concentration are M (mol/L) then use molar absorptivity e A = e× b ×c units of L/mol·cm Phenomena used for optical measurements, (1) Absorption (2) Emission (3) Luminescence (Fluorescence, Phosphorescence, Chemiluminescence (4)Scattering In all cases, response is proportional to concentration of analyte CEM 333 page 3.2
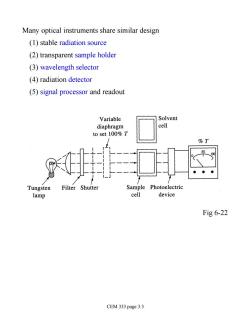
Many optical instruments share similar design (1)stable radiation source (2)transparent sample holder (3)wavelength selector (4)radiation detector (5)signal processor and readout Variable Solvent diaphragm cell to set 100%T %T ● Tungsten Filter Shutter Sample Photoelectric lamp cell device Fig 6-22 CEM 333 page 3.3
Many optical instruments share similar design (1) stable radiation source (2) transparent sample holder (3) wavelength selector (4) radiation detector (5) signal processor and readout Fig 6-22 CEM 333 page 3.3

Radiation Sources (Fig.7-3) Wavelength.nm 100 200 400 IR (a)Sources Hr Da lamp Continuur yer亿0+Y0) Nichrome wire (Ni+Cr) Globar (sic Photographic plate Photomultiplier tube Phototube Photocell Silicon diode Charge-transfer detecto Thern couple (voltage)or bolometer (resi Golay pneumatic cell CEM 333 page 3.4
Radiation Sources: (Fig. 7-3) CEM 333 page 3.4

Continuum sources produce broad range of 's(often blackbody) Heated solid (Globar,nichrome wire)(1-40 um) Tungsten lamp (300-3000 nm) Quartz Tungsten Halogen (QTH)lamp(200-3000 nm) high temperature(3500 K) Evaporation W(s)->W(g) W(g)+I2(g)→W12(g) Redeposition WI2(g)+W(s)>W(s)+I2(g) D2 lamp or Hg/Xe arc-lamp -(160-400 nm) electronic excitation D2+Eelectrical→D2*-→D(KE1)+D(KE2)+hv KE1+KE2+hv=Eelectrical-BDE bond dissociation energy CEM 333 page 3.5
Continuum sources produce broad range of l's (often blackbody) Heated solid (Globar, nichrome wire) (1-40 mm) Tungsten lamp (300-3000 nm) Quartz Tungsten Halogen (QTH) lamp (200-3000 nm) high temperature (3500 K) Evaporation W(s) ® W(g) W(g) + I2 (g) ® WI2 (g) Redeposition WI2 (g)+ W(s) ® W(s) + I2 (g) D2 lamp or Hg/Xe arc-lamp - (160-400 nm) electronic excitation D2 + Eelectrical ® D2 * ® D(KE1 ) + D(KE2 ) + hn KE1 + KE2 + hn = Eelectrical - BDE bond dissociation energy CEM 333 page 3.5
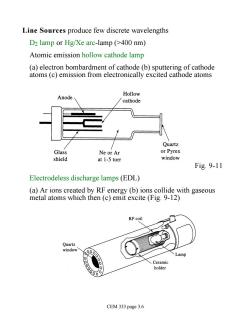
Line Sources produce few discrete wavelengths D2 lamp or Hg/Xe arc-lamp (>400 nm) Atomic emission hollow cathode lamp (a)electron bombardment of cathode (b)sputtering of cathode atoms (c)emission from electronically excited cathode atoms Hollow Anode cathode Quartz Glass Ne or Ar or Pyrex shield at 1-5 torr window Fig.9-11 Electrodeless discharge lamps(EDL) (a)Ar ions created by RF energy(b)ions collide with gaseous metal atoms which then (c)emit excite (Fig.9-12) RF coil Quartz window CEM 333 page 3.6
Line Sources produce few discrete wavelengths D2 lamp or Hg/Xe arc-lamp (>400 nm) Atomic emission hollow cathode lamp (a) electron bombardment of cathode (b) sputtering of cathode atoms (c) emission from electronically excited cathode atoms Fig. 9-11 Electrodeless discharge lamps (EDL) (a) Ar ions created by RF energy (b) ions collide with gaseous metal atoms which then (c) emit excite (Fig. 9-12) CEM 333 page 3.6

Laser Light Amplification by Stimulated Emission of Radiation (a)pumping of excited state (b)stimulated emission to produce emission (Fig.7-5) (1) (2) (3) 去去Hea Pumping energy E E Excitation Partial relaxation Metastable excited state (a)Pumping (excitation by electrical,radiant,or chemical energy) (2) (3) =E-E (b)Spontaneous emission (2) (3 (c)Stimulated emission CEM 333 page 3.7
Laser Light Amplification by Stimulated Emission of Radiation (a) pumping of excited state (b) stimulated emission to produce emission (Fig. 7-5) CEM 333 page 3.7
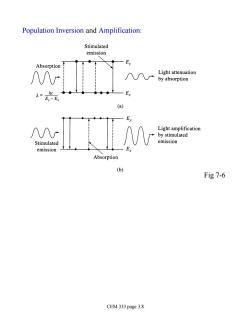
Population Inversion and Amplification: Stimulated emission Absorption Ey Light attenuation by absorption ●●● -Ex (a) Ey Light amplification by stimulated Stimulated emission emission Absorption 6 Fig 7-6 CEM 333 page 3.8
Population Inversion and Amplification: Fig 7-6 CEM 333 page 3.8
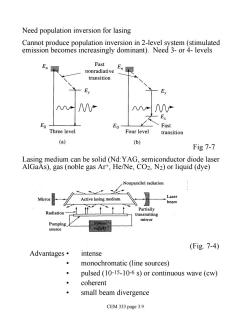
Need population inversion for lasing Cannot produce population inversion in 2-level system (stimulated emission becomes increasingly dominant).Need 3-or 4-levels En Fast En nonradiative transition ∧八 Ex Eo E01 Fast Three level Four level transition (a) (b) Fig 7-7 Lasing medium can be solid (Nd:YAG,semiconductor diode laser AlGaAs),gas(noble gas Ar+,He/Ne,CO2,N2)or liquid(dye) Nonparallel radiation . Lase Mirro Active lasing medium heam Partially Radiation transmitting mirror upply (Fig.7-4) Advantages· intense monochromatic (line sources) pulsed (10-15-10-6 s)or continuous wave(cw) coherent small beam divergence CEM 333 page 3.9
Need population inversion for lasing Cannot produce population inversion in 2-level system (stimulated emission becomes increasingly dominant). Need 3- or 4- levels Fig 7-7 Lasing medium can be solid (Nd:YAG, semiconductor diode laser AlGaAs), gas (noble gas Ar+, He/Ne, CO2, N2) or liquid (dye) (Fig. 7-4) Advantages • intense • monochromatic (line sources) • pulsed (10-15-10-6 s) or continuous wave (cw) • coherent • small beam divergence CEM 333 page 3.9

Wavelength Selectors: (Fig.7-2) Wavelength,nm 100 200 70010002000 4000700010.00020.00040.000 Spectral region VAC UV Visible NEAR IR R FAR IR (a)Ma for LE. lenses,and Fused silica or quartz prisms Corex glass Silicate glass KBr TIBr or TII Fluorite prism Fused silica or quartz prism Continuum KBr pris 3000 lines/mm Gratings 50 lines/mm Interference wedge Interference filter Discontinuous Glass fi CEM 333 page 3.10
Wavelength Selectors: (Fig. 7-2) CEM 333 page 3.10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)02 Introduction to Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)01 Instrumental Analysis.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十五章 糖类化合物 Saccharides.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十三章 杂环化合物和生物碱 Heterocyclic compounds and alkaloids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十二章 含氮和含磷有机化合物 Nitrogenous and phosphorous compounds.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十一章 取代酸 Substituted acids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十章 羧酸及其衍生物 Carboxylic Acids and Carboxylic Acid Derivatives.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第九章 醛酮醌(Aldehydes、Ketones、quinones).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第八章 醇酚醚(Alcohols、Phenols Ethers).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第七章 卤代烃 Halohydrocarbons.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第五章 旋光异构 Optical isomerism.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第四章 芳香烃 Aromatic Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第三章 不饱和烃 Unsaturated Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第二章 饱和烃(Saturated Hydrocarbon).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)绪论 Introduce Introduction.pdf
- 《无机及分析化学》课程教案讲义(打印版)10 吸光光度法.pdf
- 《无机及分析化学》课程教案讲义(打印版)09 氧化还原平衡与氧化还原滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)08 配位平衡与配位滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)07 沉淀溶解平衡和沉淀分析法.pdf
- 《无机及分析化学》课程教案讲义(打印版)06 酸碱平衡和酸碱滴定法.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)04 UV-Vis(Absorption)Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)05 Luminescence Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)06 Infrared Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)07 Introduction to Atomic Optical Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)08 Atomic Emission Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)09 Atomic Absorption Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)10 Introduction to Electrochemistry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)11 Potentiometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)12 Voltammetry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)13 Flow Injection Analysis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)14 Introduction to Chromatographic Separations.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)15_Gas Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)16_Liquid Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)17_Electrophoresis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)18_Mass Spectrometry.pdf
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第1节概述.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第2节高效毛细管电泳的理论基础.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第3节高效毛细管电泳仪.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第4节高效毛细管电泳分离模式.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第5节应用与进展.ppt
