《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)05 Luminescence Spectroscopy
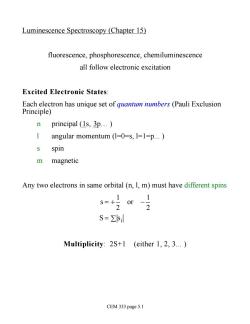
Luminescence Spectroscopy (Chapter 15) fluorescence,phosphorescence,chemiluminescence all follow electronic excitation Excited Electronic States: Each electron has unique set of quantum numbers (Pauli Exclusion Principle) n principal (1s,3p. 1 angular momentum (1=0=s,1=1=p.) s spin m magnetic Any two electrons in same orbital(n,1,m)must have different spins S=Zsil Multiplicity:2S+1 (either 1,2,3.) CEM 333 page 5.1
Luminescence Spectroscopy (Chapter 15) fluorescence, phosphorescence, chemiluminescence all follow electronic excitation Excited Electronic States: Each electron has unique set of quantum numbers (Pauli Exclusion Principle) n principal (1s, 3p. ) l angular momentum (l=0=s, l=1=p. ) s spin m magnetic Any two electrons in same orbital (n, l, m) must have different spins s = + 1 2 or - 1 2 S = s å i Multiplicity: 2S+1 (either 1, 2, 3. ) CEM 333 page 5.1
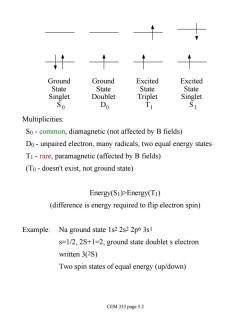
Ground Ground Excited Excited State State State State Singlet Doublet Triplet Singlet So Do Multiplicities: So-common,diamagnetic(not affected by B fields) Do-unpaired electron,many radicals,two equal energy states T1-rare,paramagnetic (affected by B fields) (To-doesn't exist,not ground state) Energy(S1)>Energy(T1) (difference is energy required to flip electron spin) Example:Na ground state 1s2 2s2 2p6 3s s=1/2,2S+1=2,ground state doublet s electron written 3(2S) Two spin states of equal energy (up/down) CEM 333 page 5.2
Ground State Singlet S Ground State Doublet D Excited State Singlet S Excited State Triplet T 0 0 1 1 Multiplicities: S0 - common, diamagnetic (not affected by B fields) D0 - unpaired electron, many radicals, two equal energy states T1 - rare, paramagnetic (affected by B fields) (T0 - doesn't exist, not ground state) Energy(S1)>Energy(T1) (difference is energy required to flip electron spin) Example: Na ground state 1s2 2s2 2p6 3s1 s=1/2, 2S+1=2, ground state doublet s electron written 3(2S) Two spin states of equal energy (up/down) CEM 333 page 5.2

Na Ist excited state 1s2 2s2 2p6 3pl D1 written 3(2P) BUT two spins states? J(total ang.mom)=L+S or L-S now1s22s22p63s1=3(2P12)and3(2P32) Term Symbol 2S+ILJ Na 3p->3s fluorescence two lines at 5896 nm (2P3/2)and 589.0 nm (2P1/2) (SEmision0S1←Absorption_S0)】 CEM 333 page 5.3
Na 1st excited state 1s2 2s2 2p6 3p1 D1 written 3(2P) BUT two spins states? J (total ang. mom)=L+S or L-S now 1s2 2s2 2p6 3s1 = 3(2P1/2) and 3(2P3/2) Term Symbol 2S+1LJ Na 3p®3s fluorescence two lines at 589.6 nm (2P3/2) and 589.0 nm (2P1/2) S1 Emission ¾ ¾ ¾ ¾ ¾ ® S0 S1 Absorption ( ¬ ¾ ¾ ¾ ¾ ¾ S0 ) CEM 333 page 5.3
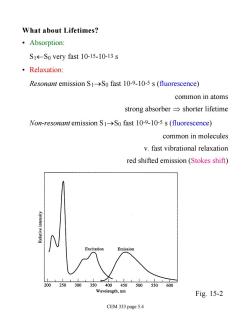
What about Lifetimes? ·Absorption: S1-So very fast 10-15-10-13 s ·Relaxation: Resonant emission S1>So fast 10-9-10-5 s(fluorescence) common in atoms strong absorber=shorter lifetime Non-resonant emission S1->So fast 10-9-10-5 s (fluorescence) common in molecules v.fast vibrational relaxation red shifted emission(Stokes shift) 200250300 Wavelength,nm Fig.15-2 CEM 333 page 5.4
What about Lifetimes? • Absorption: S1¬S0 very fast 10-15-10-13 s • Relaxation: Resonant emission S1®S0 fast 10-9-10-5 s (fluorescence) common in atoms strong absorber Þ shorter lifetime Non-resonant emission S1®S0 fast 10-9-10-5 s (fluorescence) common in molecules v. fast vibrational relaxation red shifted emission (Stokes shift) Fig. 15-2 CEM 333 page 5.4

Non-resonant emission T1->So slow 10-5-10 s(phosphorescence) Transitions between states of different multiplicities are improbable "forbidden"(e.g.T←-SorT→S) 300 400 500 600 Wavelength,nm Fig.15-3 phosphorescence fluorescence excitation CEM 333 page 5.5
Non-resonant emission T1®S0 slow 10-5-10 s (phosphorescence) Transitions between states of different multiplicities are improbable "forbidden" (e.g. T¬S or T®S) Fig. 15-3 phosphorescence fluorescence excitation CEM 333 page 5.5

Internal Conversion:radiationless transition to lower state when vibrational energy levels "match" External Conversion:radiationless transition to lower state by collisional deactivation Intersystem Crossing:transition with spin change (e.g.S to T) Fluorescence emission not involving spin change (e.g. S→S,T-→T),efficient,short-livedS), improbable,long-lived >10-5 s Dissociation excitation to vibrational state with enough energy to break bond Predissociation relaxation to state with enough energy to break bond CEM 333 page 5.6
Internal Conversion: radiationless transition to lower state when vibrational energy levels "match" External Conversion: radiationless transition to lower state by collisional deactivation Intersystem Crossing: transition with spin change (e.g. S to T) Fluorescence: emission not involving spin change (e.g. S®S, T®T), efficient, short-lived 10-5 s Dissociation: excitation to vibrational state with enough energy to break bond Predissociation: relaxation to state with enough energy to break bond CEM 333 page 5.6

Energy Level Diagram:(Fig.15-1) Singlet excited states Triplet excited state Internal Vibratio Intersystem crossing Vibrational- S ∠relaxation Groun CEM 333 page 5.7
Energy Level Diagram: (Fig. 15-1) CEM 333 page 5.7

How likely is fluorescence? Fluorescence Quantum Yield -ratio of number of molecules fluorescing to number excited photons fluor Φuor= species excited (Φhuor=0.0to1.0) Kfluor kfluor +kint con+kext con +kISC+kpre dis+kdis What Factors AffectΦuor? (l)Excitation入 Short 's break bonds increase kpre-dis and kdis rarely observed most common 0*)0元*→nπ*→π emission usually from lowest lying excited state (2)Lifetime of state Transition probability measured by s Large s implies short lifetime Largest fluorescence from short lifetime/high s state π*→π>π*→n(10-9-10-7s>10-7-10-5s) CEM 333 page 5.8
How likely is fluorescence? Fluorescence Quantum Yield - ratio of number of molecules fluorescing to number excited Ffluor = # photons fluor # species excited (Ffluor = 0.0 to 1.0) = kfluor kfluor + kint con + kext con + kISC + kpre dis + kdis What Factors Affect fluor? (1) Excitation l Short l's break bonds increase kpre-dis and kdis rarely observed most common s* ® s p* ® n p* ® p emission usually from lowest lying excited state (2) Lifetime of state Transition probability measured by e Large e implies short lifetime Largest fluorescence from short lifetime/high e state p*®p > p*®n (10-9-10-7 s > 10-7-10-5 s) CEM 333 page 5.8
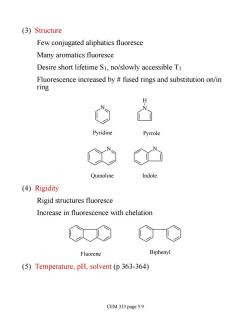
(3)Structure Few conjugated aliphatics fluoresce Many aromatics fluoresce Desire short lifetime S1,no/slowly accessible T1 Fluorescence increased by fused rings and substitution on/in ring H Pyridine Pyrrole N Quinoline Indole (4)Rigidity Rigid structures fluoresce Increase in fluorescence with chelation Fluorene Biphenyl (5)Temperature,pH,solvent(p 363-364) CEM 333 page 5.9
(3) Structure Few conjugated aliphatics fluoresce Many aromatics fluoresce Desire short lifetime S1, no/slowly accessible T1 Fluorescence increased by # fused rings and substitution on/in ring N N H N N Pyridine Pyrrole Quinoline Indole (4) Rigidity Rigid structures fluoresce Increase in fluorescence with chelation Fluorene Biphenyl (5) Temperature, pH, solvent (p 363-364) CEM 333 page 5.9

Quantitative Luminescence Spectrophotometry: Fluorescence Absorbed F =K'(10-I) =K'.2.303bcIo =K.c Only works at low A(<0.05) (i)self quenching(collisions between excited states (ii)self absorption (when absorption and fluorescence band overlap) Lamp Sample aperture Shutter disk A Primary Reference filter photomultiplie condary Sample te hotomultiplie Mirror Sample Reference aperture disk Fig.15-6 CEM 333 page 5.10
Quantitative Luminescence Spectrophotometry: F Fluorescence } = K' I( 0 - I) Absorbed 6 7 8 = K'×2.303ebc ×I 0 = K× c Only works at low A (<0.05) (i) self quenching (collisions between excited states (ii) self absorption (when absorption and fluorescence band overlap) Fig. 15-6 CEM 333 page 5.10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)04 UV-Vis(Absorption)Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)03 Optical Spectroscopy and Instrumentation.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)02 Introduction to Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)01 Instrumental Analysis.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十五章 糖类化合物 Saccharides.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十三章 杂环化合物和生物碱 Heterocyclic compounds and alkaloids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十二章 含氮和含磷有机化合物 Nitrogenous and phosphorous compounds.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十一章 取代酸 Substituted acids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十章 羧酸及其衍生物 Carboxylic Acids and Carboxylic Acid Derivatives.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第九章 醛酮醌(Aldehydes、Ketones、quinones).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第八章 醇酚醚(Alcohols、Phenols Ethers).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第七章 卤代烃 Halohydrocarbons.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第五章 旋光异构 Optical isomerism.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第四章 芳香烃 Aromatic Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第三章 不饱和烃 Unsaturated Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第二章 饱和烃(Saturated Hydrocarbon).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)绪论 Introduce Introduction.pdf
- 《无机及分析化学》课程教案讲义(打印版)10 吸光光度法.pdf
- 《无机及分析化学》课程教案讲义(打印版)09 氧化还原平衡与氧化还原滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)08 配位平衡与配位滴定法.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)06 Infrared Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)07 Introduction to Atomic Optical Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)08 Atomic Emission Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)09 Atomic Absorption Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)10 Introduction to Electrochemistry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)11 Potentiometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)12 Voltammetry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)13 Flow Injection Analysis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)14 Introduction to Chromatographic Separations.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)15_Gas Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)16_Liquid Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)17_Electrophoresis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)18_Mass Spectrometry.pdf
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第1节概述.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第2节高效毛细管电泳的理论基础.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第3节高效毛细管电泳仪.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第4节高效毛细管电泳分离模式.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第5节应用与进展.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十二章 光分析法导论 第1节光分析基础与分类.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十二章 光分析法导论 第2节原子光谱与分子光谱.ppt
