《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)01 Instrumental Analysis

CEM 333 Instrumental Analysis CEM 333 CG-MS UV-Vis AAS Fourier Transform Instruental 803H.728 Analysis Simon J.Garrett Room:CEM 234 Phone:355 9715 ext 208 E-mail:garrett@cem.msu.edu Lectures:Tuesday,Thursday 9:00-9:50 am Room 136 Office Hours:Tuesdays 10:00-11:00 am CEM 333 page 1.1
CEM 333 Instrumental Analysis Simon J. Garrett Room: CEM 234 Phone: 355 9715 ext 208 E-mail: garrett@cem.msu.edu Lectures: Tuesday, Thursday 9:00-9:50 am Room 136 Office Hours: Tuesdays 10:00-11:00 am CEM 333 page 1.1
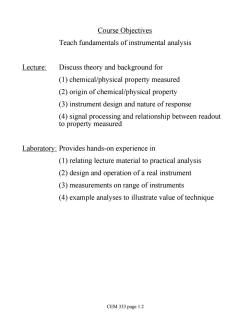
Course Objectives Teach fundamentals of instrumental analysis Lecture: Discuss theory and background for (1)chemical/physical property measured (2)origin of chemical/physical property (3)instrument design and nature of response (4)signal processing and relationship between readout to property measured Laboratory:Provides hands-on experience in (1)relating lecture material to practical analysis (2)design and operation of a real instrument (3)measurements on range of instruments (4)example analyses to illustrate value of technique CEM 333 page 1.2
Course Objectives Teach fundamentals of instrumental analysis Lecture: Discuss theory and background for (1) chemical/physical property measured (2) origin of chemical/physical property (3) instrument design and nature of response (4) signal processing and relationship between readout to property measured Laboratory: Provides hands-on experience in (1) relating lecture material to practical analysis (2) design and operation of a real instrument (3) measurements on range of instruments (4) example analyses to illustrate value of technique CEM 333 page 1.2

Introduction (Chapter 1) Classification of Analytical Methods Qualitative instrumental analysis is that measured property indicates presence of analyte in matrix Quantitative instrumental analysis is that magnitude of measured property is proportional to concentration of analyte in matrix Species of interest All constituents including analyte Matrix-analyte =concomitants Often need pretreatment-chemical extraction,distillation, separation,precipitation (A)Classical: Qualitative-identification by color,indicators,boiling points, odors Quantitative -mass or volume (e.g.gravimetric,volumetric) (B)Instrumental Qualitative -chromatography,electrophoresis and identification by measuring physical property (e.g.spectroscopy,electrode potential) Quantitative-measuring property and determining relationship to concentration(e.g.spectrophotometry,mass spectrometry) Often,same instrumental method used for qualitative and quantitative analysis CEM 333 page 1.3
Introduction (Chapter 1) Classification of Analytical Methods Qualitative instrumental analysis is that measured property indicates presence of analyte in matrix Quantitative instrumental analysis is that magnitude of measured property is proportional to concentration of analyte in matrix Species of interest All constituents including analyte. Matrix-analyte =concomitants Often need pretreatment - chemical extraction, distillation, separation, precipitation (A) Classical: Qualitative - identification by color, indicators, boiling points, odors Quantitative - mass or volume (e.g. gravimetric, volumetric) (B) Instrumental: Qualitative - chromatography, electrophoresis and identification by measuring physical property (e.g. spectroscopy, electrode potential) Quantitative - measuring property and determining relationship to concentration (e.g. spectrophotometry, mass spectrometry) Often, same instrumental method used for qualitative and quantitative analysis CEM 333 page 1.3

Types of Instrumental Methods: Property Example Method Radiation emission Emission spectroscopy -fluorescence, phosphorescence,luminescence Radiation absorption Absorption spectroscopy spectrophotometry,photometry,nuclear magnetic resonance,electron spin resonance Radiation scattering Turbidity,Raman Radiation refraction Refractometry,interferometry Radiation diffraction X-ray,electron Radiation rotation Polarimetry,circular dichroism Electrical potential Potentiometry Electrical charge Coulometry Electrical current Voltammetry-amperometry,polarography Electrical resistance Conductometry Mass Gravimetry Mass-to-charge ratio Mass spectrometry Rate of reaction Stopped flow,flow injection analysis Thermal Thermal gravimetry,calorimetry Radioactivity Activation,isotope dilution (Often combined with chromatographic or electrophoretic methods) CEM 333 page 1.4
Types of Instrumental Methods: Property Example Method Radiation emission Emission spectroscopy - fluorescence, phosphorescence, luminescence Radiation absorption Absorption spectroscopy - spectrophotometry, photometry, nuclear magnetic resonance, electron spin resonance Radiation scattering Turbidity, Raman Radiation refraction Refractometry, interferometry Radiation diffraction X-ray, electron Radiation rotation Polarimetry, circular dichroism Electrical potential Potentiometry Electrical charge Coulometry Electrical current Voltammetry - amperometry, polarography Electrical resistance Conductometry Mass Gravimetry Mass-to-charge ratio Mass spectrometry Rate of reaction Stopped flow, flow injection analysis Thermal Thermal gravimetry, calorimetry Radioactivity Activation, isotope dilution (Often combined with chromatographic or electrophoretic methods) CEM 333 page 1.4

Energy Analyte Analytical Data Stimulus in matrix) Response encoded information Example: Spectrophotometry Instrument:spectrophotometer Stimulus:monochromatic light energy Analytical response:light absorption Transducer:photocell Data:electrical current Data processor:current meter Readout:meter scale Data Domains:way of encoding analytical response in electrical or non-electrical signals. Interdomain conversions transform information from one domain to another. Light Intensity-Photocell >Current Current Metercae Detector(general):device that indicates change in environment Transducer (specific):device that converts non-electrical to electrical data Sensor(specific):device that converts chemical to electrical data CEM 333 page 1.5
Stimulus Response Analyte (in matrix) Energy Analytical Data encoded information Example: Spectrophotometry Instrument: spectrophotometer Stimulus: monochromatic light energy Analytical response: light absorption Transducer: photocell Data: electrical current Data processor: current meter Readout: meter scale Data Domains: way of encoding analytical response in electrical or non-electrical signals. Interdomain conversions transform information from one domain to another. Light Intensity Photocell ¾ ¾ ¾ ¾ ¾ ® Current Current Meter ¾ ¾ ¾ ¾ ¾ ¾ ® Scale Detector (general): device that indicates change in environment Transducer (specific): device that converts non-electrical to electrical data Sensor (specific): device that converts chemical to electrical data CEM 333 page 1.5
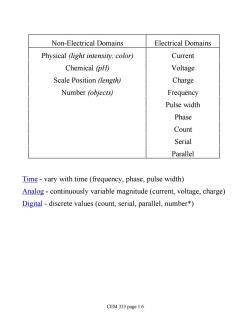
Non-Electrical Domains Electrical Domains Physical (light intensity,color) Current Chemical (pH) Voltage Scale Position (length) Charge Number (objects) Frequency Pulse width Phase Count Serial Parallel Time-vary with time(frequency,phase,pulse width) Analog-continuously variable magnitude(current,voltage,charge) Digital -discrete values (count,serial,parallel,number*) CEM 333 page 1.6
Non-Electrical Domains Electrical Domains Physical (light intensity, color) Current Chemical (pH) Voltage Scale Position (length) Charge Number (objects) Frequency Pulse width Phase Count Serial Parallel Time - vary with time (frequency, phase, pulse width) Analog - continuously variable magnitude (current, voltage, charge) Digital - discrete values (count, serial, parallel, number*) CEM 333 page 1.6
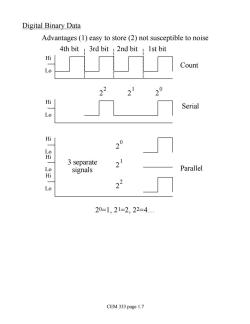
Digital Binary Data Advantages (1)easy to store(2)not susceptible to noise 4th bit 3rd bit 2nd bit 1st bit Hi Count Lo Hi Serial Lo Hi 30 品 3 separate signals Parallel Lo 23 20=1,21=2,22=4. CEM 333 page 1.7
Digital Binary Data Advantages (1) easy to store (2) not susceptible to noise 2 2 4th bit 3rd bit 2nd bit 1st bit Hi Lo Count Serial 2 0 2 1 2 0 2 1 2 2 Parallel Hi Lo Hi Lo Hi Lo Hi Lo 3 separate signals 20=1, 21=2, 22=4. CEM 333 page 1.7

Performance Characteristics:Figures of Merit How to choose an analytical method?How good is measurement? How reproducible?-Precision How close to true value?-Accuracy/Bias How small a difference can be measured?-Sensitivity What range of amounts?-Dynamic Range How much interference?-Selectivity CEM 333 page 1.8
Performance Characteristics: Figures of Merit How to choose an analytical method? How good is measurement? How reproducible? - Precision How close to true value? - Accuracy/Bias How small a difference can be measured? - Sensitivity What range of amounts? - Dynamic Range How much interference? - Selectivity CEM 333 page 1.8

Precision-Indeterminate or random errors i=N (x-x)2 Absolute standard deviation:s= N-1 Variance:s2 Relative standard deviation:RSD=S Standard deviation of mean:sm= √N Accuracy -Determinate errors (operator,method,instrumental) Bias:bias =X-Xtrue Sensitivity S=dSignal Calibration sensitivity: dc c+Signal blank mc+Signal blank (larger slope of calibration curve m,more sensitive measurement) Detection Limit Signal must be bigger than random noise of blank Minimum signal:Signal min =Av.Signal blank +ksplank From statistics k=3 or more (at 95%confidence level) CEM 333 page 1.9
Precision - Indeterminate or random errors Absolute standard deviation: s = xi ( - x ) 2 i-0 i= N å N -1 Variance: s 2 Relative standard deviation: RSD = s x Standard deviation of mean: sm = s N Accuracy - Determinate errors (operator, method, instrumental) Bias: bias = x - xtrue Sensitivity Calibration sensitivity: S = dSignal dc c + Signal blank = mc + Signal blank (larger slope of calibration curve m, more sensitive measurement) Detection Limit Signal must be bigger than random noise of blank Minimum signal: Signal min = Av. Signal blank + ksblank From statistics k=3 or more (at 95% confidence level) CEM 333 page 1.9

Dynamic Range At detection limit we can say confidently analyte is present but cannot perform reliable quantitation Level of quantitation (LOQ):k=10 Limit of linearity (LOL):when signal is no longer proportional to concentration LOL Dynamic range 102to>106 LOQ Selectivity: No analytical method is completely free from interference by concomitants.Best method is more sensitive to analyte than interfering species (interferent). Matrix with species A&B:Signal =mAcA +mBcB+Signal blank mB Selectivity coefficient:kB.A= m A k's vary between 0(no selectivity)and large number(very selective). CEM 333 page 1.10
Dynamic Range At detection limit we can say confidently analyte is present but cannot perform reliable quantitation Level of quantitation (LOQ): k=10 Limit of linearity (LOL): when signal is no longer proportional to concentration Dynamic range: LOL LOQ 102 to > 106 Selectivity: No analytical method is completely free from interference by concomitants. Best method is more sensitive to analyte than interfering species (interferent). Matrix with species A&B: Signal = mAcA + mBcB + Signal blank Selectivity coefficient: kB,A = mB mA k's vary between 0 (no selectivity) and large number (very selective). CEM 333 page 1.10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十五章 糖类化合物 Saccharides.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十三章 杂环化合物和生物碱 Heterocyclic compounds and alkaloids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十二章 含氮和含磷有机化合物 Nitrogenous and phosphorous compounds.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十一章 取代酸 Substituted acids.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第十章 羧酸及其衍生物 Carboxylic Acids and Carboxylic Acid Derivatives.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第九章 醛酮醌(Aldehydes、Ketones、quinones).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第八章 醇酚醚(Alcohols、Phenols Ethers).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第七章 卤代烃 Halohydrocarbons.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第五章 旋光异构 Optical isomerism.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第四章 芳香烃 Aromatic Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第三章 不饱和烃 Unsaturated Hydrocarbon.pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)第二章 饱和烃(Saturated Hydrocarbon).pdf
- 山东农业大学:《有机化学》课程教学资源(课件讲稿)绪论 Introduce Introduction.pdf
- 《无机及分析化学》课程教案讲义(打印版)10 吸光光度法.pdf
- 《无机及分析化学》课程教案讲义(打印版)09 氧化还原平衡与氧化还原滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)08 配位平衡与配位滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)07 沉淀溶解平衡和沉淀分析法.pdf
- 《无机及分析化学》课程教案讲义(打印版)06 酸碱平衡和酸碱滴定法.pdf
- 《无机及分析化学》课程教案讲义(打印版)05 化学分析.pdf
- 《无机及分析化学》课程教案讲义(打印版)04 物质结构.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)02 Introduction to Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)03 Optical Spectroscopy and Instrumentation.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)04 UV-Vis(Absorption)Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)05 Luminescence Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)06 Infrared Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)07 Introduction to Atomic Optical Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)08 Atomic Emission Spectroscopy.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)09 Atomic Absorption Spectrometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)10 Introduction to Electrochemistry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)11 Potentiometry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)12 Voltammetry.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)13 Flow Injection Analysis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)14 Introduction to Chromatographic Separations.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)15_Gas Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)16_Liquid Chromatography.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)17_Electrophoresis.pdf
- 《仪器分析 Instrumental Analysis》课程教学资源(教材讲义)18_Mass Spectrometry.pdf
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第1节概述.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第2节高效毛细管电泳的理论基础.ppt
- 《仪器分析》课程教学讲义(PPT课件)第十一章 高效毛细管电泳分析 第3节高效毛细管电泳仪.ppt
