西北师范大学:《仪器分析》课程教学资源(参考资料)potentoal titration the first and second derivatives_potentoal titration the first and second derivatives

[Go back to CEM 333 Course Information Page][Go back to CEM 333 Home Page] An Excel Spreadsheet Exercise* Question Use the data in the table below to construct a spreadsheet showing the titration curve data, the first-derivative data and the second-derivative data. Plot each of these results versus titrant volume and determine the end point of the titration. Volume AgNO3, mL E vs. SCE, V 5.00 0.062 15.00 0.085 20.00 0.107 22.00 0.123 24.00 0.138 23.50 0.146 23.80 0.161 24.00 0.174 24.10 0.183 24.20 0.194 24.30 0.233 24.40 0.316 24.50 0.340 24.60 0.351 24.70 0.358
[Go back to CEM 333 Course Information Page][Go back to CEM 333 Home Page] An Excel Spreadsheet Exercise* Question Use the data in the table below to construct a spreadsheet showing the titration curve data, the first-derivative data and the second-derivative data. Plot each of these results versus titrant volume and determine the end point of the titration. Volume AgNO3, mL E vs. SCE, V 5.00 0.062 15.00 0.085 20.00 0.107 22.00 0.123 24.00 0.138 23.50 0.146 23.80 0.161 24.00 0.174 24.10 0.183 24.20 0.194 24.30 0.233 24.40 0.316 24.50 0.340 24.60 0.351 24.70 0.358

25.00 0.373 25.50 0.385 26.00 0.396 28.00 0.426 Answer We will first enter the data into the spreadsheet and construct an ordinary titration curve. Enter the data from the table above and use the Chart Wizard to plot the ordinary titration curve as shown in Figure 1. This plot is made as an XY(scatter) plot. Name the Workbook, POTTITR.xls and save it. Figure 1. Now we will compute the first derivative. In order to make room for these to line up with the appropriate volumes let us copy the data (columns A and B from Figure 1) into a separate sheet, Sheet2, leaving the original data and plot in Sheet1. Now, in Sheet 2, insert blank rows between each pair of data points as shown in Figure 2. Label columns C, D, E, and F with "[Delta]E, V", "[Delta]V, mL", "[Delta]E/[Delta]V, V/mL" and "[Delta]2 E/[Delta]V2 , V2 /mL2 " respectively. The first derivative between the first two points (5.00 and 15.00 mL) is the difference in cell potential [Delta]E = (0.085 - 0.062) V divided by the difference in volume [Delta]V = (15.00-5.00) ml. For cell C3, calculate [Delta]E as =B4-B2 and
25.00 0.373 25.50 0.385 26.00 0.396 28.00 0.426 Answer We will first enter the data into the spreadsheet and construct an ordinary titration curve. Enter the data from the table above and use the Chart Wizard to plot the ordinary titration curve as shown in Figure 1. This plot is made as an XY(scatter) plot. Name the Workbook, POTTITR.xls and save it. Figure 1. Now we will compute the first derivative. In order to make room for these to line up with the appropriate volumes let us copy the data (columns A and B from Figure 1) into a separate sheet, Sheet2, leaving the original data and plot in Sheet1. Now, in Sheet 2, insert blank rows between each pair of data points as shown in Figure 2. Label columns C, D, E, and F with "[Delta]E, V", "[Delta]V, mL", "[Delta]E/[Delta]V, V/mL" and "[Delta]2 E/[Delta]V2 , V2 /mL2 " respectively. The first derivative between the first two points (5.00 and 15.00 mL) is the difference in cell potential [Delta]E = (0.085 - 0.062) V divided by the difference in volume [Delta]V = (15.00-5.00) ml. For cell C3, calculate [Delta]E as =B4-B2 and
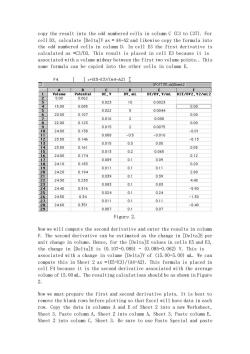
copy the result into the odd numbered cells in column C (C3 to C37). For cell D3, calculate [Delta]V as = A4-A2 and likewise copy the formula into the odd numbered cells in column D. In cell E3 the first derivative is calculated as =C3/D3. This result is placed in cell E3 because it is associated with a volume midway between the first two volume points. This same formula can be copied into the other cells in column E. Figure 2. Now we will compute the second derivative and enter the results in column F. The second derivative can be estimated as the change in [Delta]E per unit change in volume. Hence, for the [Delta]E values in cells E5 and E3, the change in [Delta]E is (0.107-0.085) - (0.085-0.062) V. This is associated with a change in volume [Delta]V of (15.00-5.00) mL. We can compute this in Sheet 2 as =(E5-E3)/(A4-A2). This formula is placed in cell F4 because it is the second derivative associated with the average volume of 15.00 mL. The resulting calculations should be as shown in Figure 2. Now we must prepare the first and second derivative plots. It is best to remove the blank rows before plotting so that Excel will have data in each row. Copy the data in columns A and E of Sheet 2 into a new Worksheet, Sheet 3. Paste column A, Sheet 2 into column A, Sheet 3. Paste column E, Sheet 2 into column C, Sheet 3. Be sure to use Paste Special and paste
copy the result into the odd numbered cells in column C (C3 to C37). For cell D3, calculate [Delta]V as = A4-A2 and likewise copy the formula into the odd numbered cells in column D. In cell E3 the first derivative is calculated as =C3/D3. This result is placed in cell E3 because it is associated with a volume midway between the first two volume points. This same formula can be copied into the other cells in column E. Figure 2. Now we will compute the second derivative and enter the results in column F. The second derivative can be estimated as the change in [Delta]E per unit change in volume. Hence, for the [Delta]E values in cells E5 and E3, the change in [Delta]E is (0.107-0.085) - (0.085-0.062) V. This is associated with a change in volume [Delta]V of (15.00-5.00) mL. We can compute this in Sheet 2 as =(E5-E3)/(A4-A2). This formula is placed in cell F4 because it is the second derivative associated with the average volume of 15.00 mL. The resulting calculations should be as shown in Figure 2. Now we must prepare the first and second derivative plots. It is best to remove the blank rows before plotting so that Excel will have data in each row. Copy the data in columns A and E of Sheet 2 into a new Worksheet, Sheet 3. Paste column A, Sheet 2 into column A, Sheet 3. Paste column E, Sheet 2 into column C, Sheet 3. Be sure to use Paste Special and paste
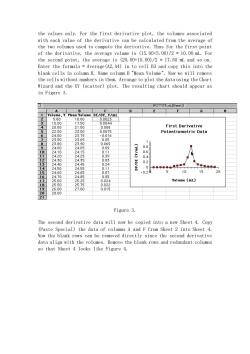
the values only. For the first derivative plot, the volumes associated with each value of the derivative can be calculated from the average of the two volumes used to compute the derivative. Thus for the first point of the derivative, the average volume is (15.00+5.00)/2 = 10.00 mL. For the second point, the average is (20.00+15.00)/2 = 17.50 mL and so on. Enter the formula = Average(A2,A4) in to cell B3 and copy this into the blank cells in column B. Name column B "Mean Volume". Now we will remove the cells without numbers in them. Arrange to plot the data using the Chart Wizard and the XY (scatter) plot. The resulting chart should appear as in Figure 3. Figure 3. The second derivative data will now be copied into a new Sheet 4. Copy (Paste Special) the data of columns A and F from Sheet 2 into Sheet 4. Now the blank rows can be removed directly since the second derivative data align with the volumes. Remove the blank rows and redundant columns so that Sheet 4 looks like Figure 4
the values only. For the first derivative plot, the volumes associated with each value of the derivative can be calculated from the average of the two volumes used to compute the derivative. Thus for the first point of the derivative, the average volume is (15.00+5.00)/2 = 10.00 mL. For the second point, the average is (20.00+15.00)/2 = 17.50 mL and so on. Enter the formula = Average(A2,A4) in to cell B3 and copy this into the blank cells in column B. Name column B "Mean Volume". Now we will remove the cells without numbers in them. Arrange to plot the data using the Chart Wizard and the XY (scatter) plot. The resulting chart should appear as in Figure 3. Figure 3. The second derivative data will now be copied into a new Sheet 4. Copy (Paste Special) the data of columns A and F from Sheet 2 into Sheet 4. Now the blank rows can be removed directly since the second derivative data align with the volumes. Remove the blank rows and redundant columns so that Sheet 4 looks like Figure 4

Figure 4. Plot these data using the Chart Wizard to obtain the plot shown on the right side of the figure. If you would like to determine the end point very precisely, you can manipulate the axes to locate the equivalence point. Here the end point is seen to be approximately 24.33 mL. This could have also been done on the first derivative plot, but it is somewhat easier to find the zero crossing than it is to find the maximum. *Courtesy Prof. S.R. Crouch
Figure 4. Plot these data using the Chart Wizard to obtain the plot shown on the right side of the figure. If you would like to determine the end point very precisely, you can manipulate the axes to locate the equivalence point. Here the end point is seen to be approximately 24.33 mL. This could have also been done on the first derivative plot, but it is somewhat easier to find the zero crossing than it is to find the maximum. *Courtesy Prof. S.R. Crouch
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 西北师范大学:《仪器分析》课程教学资源(参考资料)电位滴定法中终点电位的确定方法.doc
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第九章 紫外光谱法(Ultraviolet Spectrophotometry, UV).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第八章 原子吸收光谱法(Atomic Absorption Spectrometry, AAS).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第七章 原子发射光谱法(Atomic Emission Spectrometry, AES).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第六章 电解与库仑分析法.ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第五章 伏安分析法(Voltammetry).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第四章 电位分析法(Potentiometry).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第三章 高效液相色谱(High Pertormance Liquid Chromatography,HPLC).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第二章 气相色谱法.ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第一章 绪论 INSTRUMENTAL ANALYSIS(主讲:马永钧).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)第十章 红外吸收光谱法(Infrared Absorption Spectroscopy, IR).ppt
- 西北师范大学:《仪器分析》课程教学资源(参考资料)Detectores Detectores.pdf
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 09 紫外吸收光谱法(Ultraviolet Spectrophotometry, UV).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 08 原子吸收法 Atomic absorption spectrometry(AAS).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 07 原子发射法(Atomic Emission Spectrometry, AES).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 04 电位法 potentiometry.ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 03 High Performance Liquid Chromatography(HPLC).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 02 气相色谱法 Gas Chromatography(GC).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 12 质谱分析(Mass Spectrometry, MS).ppt
- 西北师范大学:《仪器分析》课程教学资源(PPT课件)Chapter 11 核磁共振波谱法(Nuclear magnetic resonance spectroscopy,NMR).ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十章 醇和醚.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十一章 酚.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十二章 醛、酮.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十三章 羧酸及其衍生物.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十四章 β-二羰基化合物.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十五章 胺.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十六章 重氮化合物和偶氮化合物.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十七章 杂环化合物.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十八章 碳水化合物.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第十九章 氨基酸和蛋白质.ppt
- 天津科技大学:《有机化学》课程教学资源(教案讲义)有机化学实验指导书.doc
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第一章 有机化学绪论.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第二章 烷烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第三章 烯烃和二烯烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第四章 炔烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第五章 脂环烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第六章 芳香烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第七章 稠环芳烃.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第八章 对映异构.ppt
- 天津科技大学:《有机化学》课程教学资源(PPT课件)第九章 卤代烃.ppt
