《病原生物学(医学微生物学)》课程教学课件(英文)03_Cultivation of Microorganisms

Section IFundaments of MicrobiologyCultivation of MicroorganismsSHIHEZI UNIVERSITY
Section I Fundaments of Microbiology Cultivation of Microorganisms SHIHEZI UNIVERSITY
KEY TERMSHalopilicObligateaerobeOsmophilicObligate anaerobeMediumAerotolerant anaerobeFacultative anaerobeMicroaerophilicMesophileThermophilePsychrophileSHIHEZI UNIVERSITY
KEY TERMS Obligate aerobe Obligate anaerobe Aerotolerant anaerobe Facultative anaerobe Microaerophilic Mesophile Thermophile Psychrophile Halophilic Osmophilic Medium SHIHEZI UNIVERSITY

Bacterial Requirements forGrowth? Nutrients? Oxygen (or absence). Energy· Optimal temperature? Optimal pHSHIHEZI UNIVERSITY
Bacterial Requirements for Growth • Nutrients • Oxygen (or absence) • Energy • Optimal temperature • Optimal pH SHIHEZI UNIVERSITY

SOURCES OF METABOLICENERGY The three major mechanisms for generatingmetabolic energy areFermentationRespirationPhotosynthesis At least one of these mechanisms must beemployed if an organism is to growSHIHEZI UNIVERSITY
SOURCES OF METABOLIC ENERGY The three major mechanisms for generating metabolic energy are Fermentation Respiration Photosynthesis At least one of these mechanisms must be employed if an organism is to grow SHIHEZI UNIVERSITY

FermentationThe formation of ATP in fermentation is not coupled tothe transfer of electronsFermentation is a substrate phosphorylation, anenzymatic process in which a pyrophosphate bond isdonated directly to ADP (adenosine diphosphate) by aphosphorylated metabolic intermediate The phosphorylated intermediates are formed bymetabolic rearrangement of a fermentable substrate suchas glucose, lactose, or arginineExample: fermentation of a molecule of glucose(C6H1206) yields a net gain of two pyrophosphatebonds in ATP and produces two molecules of pyruvicacid (C3H603)SHIHEZI UNIVERSITY
Fermentation The formation of ATP in fermentation is not coupled to the transfer of electrons Fermentation is a substrate phosphorylation, an enzymatic process in which a pyrophosphate bond is donated directly to ADP (adenosine diphosphate) by a phosphorylated metabolic intermediate The phosphorylated intermediates are formed by metabolic rearrangement of a fermentable substrate such as glucose, lactose, or arginine Example: fermentation of a molecule of glucose (C6H12O6) yields a net gain of two pyrophosphate bonds in ATP and produces two molecules of pyruvic acid (C3H6O3) SHIHEZI UNIVERSITY
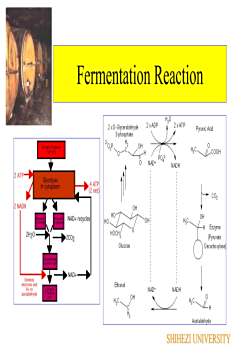
Fermentation ReactionH,02 xATP2 xADP2 × D-GlyceraldehydePyruvic Acid3-phosphateOHGuose (6 Camon)CGH12000FPO.30NAD+NADH2ATP-Glycolysis.4ATPIncytoplasm(2 net)+CO22NADHOHOHNAD+ recyclesmaOHH.C.HO中-HEnzymeHOCH,2H20(Pyruvate2C02Gluco seDecarboxylaseNAD+DonatesEthanolelectrons andNAD+NADHH+toELOIDCarborsacetaldehydeOHDedTAcetaldehydeSHIHEZI UNIVERSITY
Fermentation Reaction SHIHEZI UNIVERSITY

RespirationC.H20 + 02RespirationCO2 + H2OEneraRespiration is chemical reduction of an oxidantelectron acceptor) through a specific series of electroncarriers in the membrane establishes the proton motiveforce across the bacterial membraneReductants (electron donor)may be organic or inorganic: For example, lactic acid serves as areductant for some organisms, and hydrogen gas is a reductant forother organismsOxidants+ Gaseous oxygen (O,) often is employed as an oxidant butalternative oxidants that are employed by some organisms includecarbon dioxide (CO,), sulfate (SO 2), and nitrate (NO,SHIHEZIUNIVERSITY
Respiration Respiration is chemical reduction of an oxidant (electron acceptor) through a specific series of electron carriers in the membrane establishes the proton motive force across the bacterial membrane Reductants (electron donor) may be organic or inorganic: For example, lactic acid serves as a reductant for some organisms, and hydrogen gas is a reductant for other organisms Oxidants Gaseous oxygen (O2) often is employed as an oxidant, but alternative oxidants that are employed by some organisms include carbon dioxide (CO2), sulfate (SO4 2–), and nitrate (NO3 −) SHIHEZI UNIVERSITY
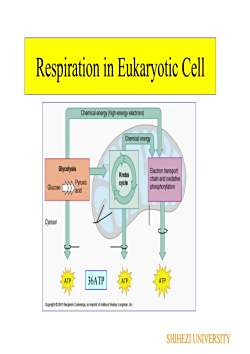
Respiration in Eukaryotic CellChemicalenergy(high-energyelectrons)ChemicalenergyGlycolysisElectron transportKrebschain and oxidativePyruviccycleGlucosephosphorylationacidVCytosol36ATPATEnmySHIHEZI UNIVERSITY
Respiration in Eukaryotic Cell 36ATP SHIHEZI UNIVERSITY
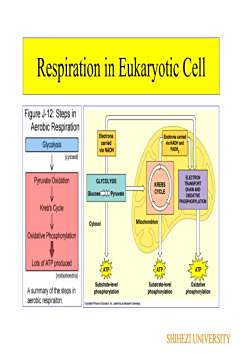
Respiration in Eukaryotic CellFigure J-12: Steps inAerobic RespirationElectronsElectrons carriedcarriedvia NADH andGlycolysisFADH,via NADH(cytoso)ELECTRONPyruvate OxidationGLYCOLYSISTRANSPORTKREBSCHAINANDCYCLEGlucosePyruvateOXIDATIVEPHOSPHORYLATIONKreb's CycleMitochondrionCytosolOxidativePhosphorylatlonLots of ATP producedATPATP(mitochondria)OxidativeSubstrate-levelSubstrate-levelA summary of the steps inphosphorylationphosphorylationphosphorylationaerobic respiraiton.SHIHEZI UNIVERSITY
Respiration in Eukaryotic Cell SHIHEZI UNIVERSITY

PHOTOSYTHENSISWATER+LIGHT-CHEMICALENERGY1.Chioroplaststrp Igt eneg2.WiterentersleafPhotosynthesisLight energyPhotosynthesis is similar to respiration in thatthe reduction of an oxidant via a specific series ofelectron carriers establishes the proton motiveforceDifference in the two processesin photosynthesis the reductant and oxidant arecreated photochemically by light energy absorbedby pigments in the membranePhotosynthesis can continue only as long as thereis a source of light energySHIHEZI UNIVERSITY
Photosynthesis Photosynthesis is similar to respiration in that the reduction of an oxidant via a specific series of electron carriers establishes the proton motive force Difference in the two processes in photosynthesis the reductant and oxidant are created photochemically by light energy absorbed by pigments in the membrane Photosynthesis can continue only as long as there is a source of light energy SHIHEZI UNIVERSITY
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《病原生物学(医学微生物学)》课程教学课件(英文)02_Bacteria Growth_Survival_Death_01.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)08_Spore-forming G+ bacilli_Clostridia.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)05_Bacteria Genetics.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)04_Microbial Metabolism.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)06_Bacteria Pathogenesis.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)07_Normal microbial flora.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)13_Enterobacteriaceae.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)12_Nesseria.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)10_Staphylococcus.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)18_Virio_Vampylo_Helico.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)17_Pseudomonoads_acinetobacter.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)19A_Haemophilus_Bordetella.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)22_Lab Diagnosis for Bacterial Infections.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)21_Non-spore-forming G+ bacilli_Corynebacterium_Listeria_Actinomycetes.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)20_Yersinia_Pasteurella.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)19B_Brucella_Francisella.pdf
- 《病原生物学(医学微生物学)》课程教学课件(讲稿)01 绪论.pdf
- 《病原生物学(医学微生物学)》课程教学课件(讲稿)02 细菌形态结构.pdf
- 《病原生物学(医学微生物学)》课程教学课件(讲稿)09-1 微生物感染的病原学检查法.pdf
- 《病原生物学(医学微生物学)》课程教学课件(讲稿)03 细菌生理.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)03_BacterialPhysiology.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)00_Introduction.pdf
- 《病原生物学(医学微生物学)》课程教学课件(英文)01_BacterialStructure.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第36章 真菌学总论.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第35章 阮粒(Prion).pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第37章 主要病原性真菌.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第34章 其他病毒.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第33章 反转录病毒.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第28章 急性胃肠炎病毒(Acute gastroenteritis virus).pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第27章 肠道病毒 enterovirus.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第30章 虫媒病毒.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第32章 疱疹病毒.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第29章 肝炎病毒.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第26章 呼吸道病毒 Viruses associated with respiratory infections.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第24章 病毒的感染与免疫.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第23章 病毒学.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第25章 病毒感染的检查方法与防治原则.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第22章 螺旋体.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第20章 立克次体.pdf
- 《病原生物学(医学微生物学)》课程教学课件(2012)第18章 放线菌属与诺卡菌属.pdf
