《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Minerals

MINERALS Ashing A. Dry Ashing 1. 550oC until light-gray ash or to constant wt. 2. Cool in dessicator 3. Weigh soon after reaching room temperature
MINERALS Ashing A. Dry Ashing 1. 550oC until light-gray ash or to constant wt. 2. Cool in dessicator 3. Weigh soon after reaching room temperature

Advantages: 1. Safe 2. Multiple samples 3. Only oven and dishes needed Disadvantages: Contamination
Advantages: 1. Safe 2. Multiple samples 3. Only oven and dishes needed Disadvantages: Contamination

B. Wet Ashing Oxidation of organic compounds Time: Completely oxidized Advantages: Complete digestion Disadvantages: 1. Explosion or corrosion 2. Requires time
B. Wet Ashing Oxidation of organic compounds Time: Completely oxidized Advantages: Complete digestion Disadvantages: 1. Explosion or corrosion 2. Requires time

Step 1: Ashing Step 2: Solublize ash in Conc. HCl - boil and evaporate solution to dryness. Step 3: Re-dissolve residue in 0.5 N HCl. Step 4: Conc. or dilute as desired Step 5: Determination of individual components. Elemental Analysis
Step 1: Ashing Step 2: Solublize ash in Conc. HCl - boil and evaporate solution to dryness. Step 3: Re-dissolve residue in 0.5 N HCl. Step 4: Conc. or dilute as desired Step 5: Determination of individual components. Elemental Analysis

1. Spectrometric Methods 2. Emission Spectroscopy - Flame Photometry Method 3. Atomic Absorption Spectrometry METHODS FOR DETERMINING MINERAL CONSTITUENTS
1. Spectrometric Methods 2. Emission Spectroscopy - Flame Photometry Method 3. Atomic Absorption Spectrometry METHODS FOR DETERMINING MINERAL CONSTITUENTS

Formation of colored complex with some ligand. Example: Fe++ (Ferrous Ion) with phenanthroline (orthophenanthrolines). 1. Spectrophotometric Method N N Fe++ .
Formation of colored complex with some ligand. Example: Fe++ (Ferrous Ion) with phenanthroline (orthophenanthrolines). 1. Spectrophotometric Method N N Fe++ .

A pair of unshared electrons can coordinate certain metallic ions to give complexes. In the case of ferrous ion, the orthophenanthroline complex is quite stable and is intensely red in color. The complex is sometimes called Ferroin
A pair of unshared electrons can coordinate certain metallic ions to give complexes. In the case of ferrous ion, the orthophenanthroline complex is quite stable and is intensely red in color. The complex is sometimes called Ferroin

Measure the intensity of emitted radiation 2. Flame Emission Spectroscopy Ground State Excited State Emits Special Electromagnetic Radiation
Measure the intensity of emitted radiation 2. Flame Emission Spectroscopy Ground State Excited State Emits Special Electromagnetic Radiation
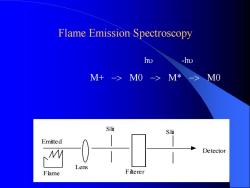
Flame Emission Spectroscopy Flame Lens Slit Filterer Slit Detector Emitted hu -hu M+ -> M0 -> M* -> M0
Flame Emission Spectroscopy Flame Lens Slit Filterer Slit Detector Emitted hu -hu M+ -> M0 -> M* -> M0
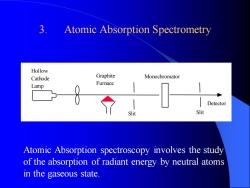
3. Atomic Absorption Spectrometry Hollow Cathode Lamp Graphite Furnace Slit Monochromator Slit Detector Atomic Absorption spectroscopy involves the study of the absorption of radiant energy by neutral atoms in the gaseous state
3. Atomic Absorption Spectrometry Hollow Cathode Lamp Graphite Furnace Slit Monochromator Slit Detector Atomic Absorption spectroscopy involves the study of the absorption of radiant energy by neutral atoms in the gaseous state
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Carbohydrate.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Spectroscopy.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Water.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Gas Liquid Chromatography.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Liquid Chromatography.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Protein.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Physical State of Ingredients in Food Systems.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Introduction.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Lipid.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Vitamin.ppt
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Enzyme Kinetics.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Primary sequence.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Protein Denaturation.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Modifications of Food Proteins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Protein Functionality.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Proteins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Chemical Kinetics.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Water activity and toxins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Introduction.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Enzymes.pdf
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Lipids and Fats.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Water.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Experimental Foods.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Top Ten Food Companies Public and Private Companies.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Objective Evaluation of Food.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)General Food Safety.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Carbohydrates:Starch and Sugars.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Preservation.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Meat.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Milk and Milk Products.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Eggs.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Flour.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Enzymes.ppt
- 华东理工大学:《物理化学》课程教学资源(课件讲稿)绪论 Physical Chemistry.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)00 Chapter 0 Introduction.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)01 Chapter 1 The properties of gases.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)02 Chapter 2 The First Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)03 Chapter 3 The First Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)04 br Chapter 4 The Second Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)05 Chapter 5 The Second Law the machinery.pdf
