华东理工大学:《物理化学》课程教学课件(讲稿,英文版)01 Chapter 1 The properties of gases

20 版权所有:华东理工大学物理化学教研室 上一页 下一页 返回目录 Part 1: Equilibrium Bilingual Program 1. The properties of gases
20 版权所有:华东理工大学物理化学教研室 上一页 下一页 返回目录 Part 1: Equilibrium Bilingual Program 1. The properties of gases

版权所有:华东理工大学物理化学教研室 21 This chapter establishes the properties of gases that will be used throughout the text. It begins with an account of an idealized version of a gas, a perfect gas, and shows how its equation of state may be assembled experimentally. We then see how this relation between the properties of the gas can be explained in terms of the kinetic model, in which the gas is represented by a collection of point masses in continuous random motion. Finally, we see how the properties of real gases differ from those of a perfect gas, a n d c o n s t r u c t an equation of state that describes their properties. 1. The properties of gases
版权所有:华东理工大学物理化学教研室 21 This chapter establishes the properties of gases that will be used throughout the text. It begins with an account of an idealized version of a gas, a perfect gas, and shows how its equation of state may be assembled experimentally. We then see how this relation between the properties of the gas can be explained in terms of the kinetic model, in which the gas is represented by a collection of point masses in continuous random motion. Finally, we see how the properties of real gases differ from those of a perfect gas, a n d c o n s t r u c t an equation of state that describes their properties. 1. The properties of gases
版权所有:华东理工大学物理化学教研室 22 The perfect gas 1.1 The states of gases 1.2 The gas laws 1.3 The kinetic model of gases Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states 1. The properties of gases
版权所有:华东理工大学物理化学教研室 22 The perfect gas 1.1 The states of gases 1.2 The gas laws 1.3 The kinetic model of gases Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states 1. The properties of gases

版权所有:华东理工大学物理化学教研室 23 The perfect gas The simplest state of matter is a gas, a form of matter that fills any container it occupies. A gas may be pictured as a collection of molecules in continuous random motion, with speeds that increase as the temperature is raised. A gas differs from a liquid in that the molecules of a gas are widely separated from one another and move in paths t h a t a r e l a r g e l y unaffected by intermolecular forces. 1. The properties of gases
版权所有:华东理工大学物理化学教研室 23 The perfect gas The simplest state of matter is a gas, a form of matter that fills any container it occupies. A gas may be pictured as a collection of molecules in continuous random motion, with speeds that increase as the temperature is raised. A gas differs from a liquid in that the molecules of a gas are widely separated from one another and move in paths t h a t a r e l a r g e l y unaffected by intermolecular forces. 1. The properties of gases
版权所有:华东理工大学物理化学教研室 24 1.1 The states of gases The physical state of a sample of a substance is defined by its physical properties, and two samples of a substance that have the same physical properties are in the same state.The state of a pure gas is specified by giving the values of its volume, V, amount of substance, n, pressure, p, and temp-erature, T. However, it has been established experimentally that it is sufficient to specify only three of these variables, for then the fourth variable is fixed. That is, it is an experi-mental fact that each substance is described by an equation of state, an equation that interrelates these four variables. The general form of an equation of state is: p= f (T,V,n)
版权所有:华东理工大学物理化学教研室 24 1.1 The states of gases The physical state of a sample of a substance is defined by its physical properties, and two samples of a substance that have the same physical properties are in the same state.The state of a pure gas is specified by giving the values of its volume, V, amount of substance, n, pressure, p, and temp-erature, T. However, it has been established experimentally that it is sufficient to specify only three of these variables, for then the fourth variable is fixed. That is, it is an experi-mental fact that each substance is described by an equation of state, an equation that interrelates these four variables. The general form of an equation of state is: p= f (T,V,n)

版权所有:华东理工大学物理化学教研室 25 1). Pressure, p Definition: Pressure is defined as force divided by the area to which the force is applied. Unit: Pascal (Pa), 1 Pa= 1 Nm-2; the SI unit. It is defined as 1 newton per meter squared; Standard pressure: , op 10 Pa 5 = o p 1.1 The states of gases The general form of an equation of state is: p= f (T,V,n)
版权所有:华东理工大学物理化学教研室 25 1). Pressure, p Definition: Pressure is defined as force divided by the area to which the force is applied. Unit: Pascal (Pa), 1 Pa= 1 Nm-2; the SI unit. It is defined as 1 newton per meter squared; Standard pressure: , op 10 Pa 5 = o p 1.1 The states of gases The general form of an equation of state is: p= f (T,V,n)
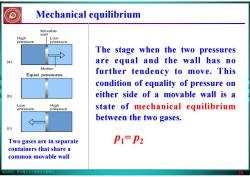
版权所有:华东理工大学物理化学教研室 26 Mechanical equilibrium Two gases are in separate containers that share a common movable wall The stage when the two pressures are equal and the wall has no further tendency to move. This condition of equality of pressure on either side of a movable wall is a state of mechanical equilibrium between the two gases. p1= p2
版权所有:华东理工大学物理化学教研室 26 Mechanical equilibrium Two gases are in separate containers that share a common movable wall The stage when the two pressures are equal and the wall has no further tendency to move. This condition of equality of pressure on either side of a movable wall is a state of mechanical equilibrium between the two gases. p1= p2

版权所有:华东理工大学物理化学教研室 27 2). Temperature, T Temperature scales: • The Celsius scale of temperature, θ, ℃ • The thermodynamic temperature scale, T, K T / K= θ, / ℃+273.15 The temperature is the property that tells us the direction of the flow of energy, 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 27 2). Temperature, T Temperature scales: • The Celsius scale of temperature, θ, ℃ • The thermodynamic temperature scale, T, K T / K= θ, / ℃+273.15 The temperature is the property that tells us the direction of the flow of energy, 1.1 The states of gases

版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases
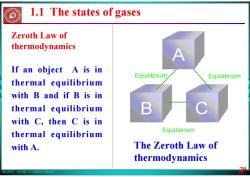
版权所有:华东理工大学物理化学教研室 29 The Zeroth Law of thermodynamics Zeroth Law of thermodynamics If an object A is in thermal equilibrium with B and if B is in thermal equilibrium with C, then C is in thermal equilibrium with A. 29 1.1 The states of gases
版权所有:华东理工大学物理化学教研室 29 The Zeroth Law of thermodynamics Zeroth Law of thermodynamics If an object A is in thermal equilibrium with B and if B is in thermal equilibrium with C, then C is in thermal equilibrium with A. 29 1.1 The states of gases
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)00 Chapter 0 Introduction.pdf
- 华东理工大学:《物理化学》课程教学资源(课件讲稿)绪论 Physical Chemistry.pdf
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Enzymes.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Flour.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Eggs.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Milk and Milk Products.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Meat.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Preservation.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Carbohydrates:Starch and Sugars.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)General Food Safety.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Objective Evaluation of Food.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Top Ten Food Companies Public and Private Companies.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Experimental Foods.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Water.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Lipids and Fats.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Minerals.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Carbohydrate.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Spectroscopy.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Water.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Gas Liquid Chromatography.ppt
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)02 Chapter 2 The First Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)03 Chapter 3 The First Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)04 br Chapter 4 The Second Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)05 Chapter 5 The Second Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)06 Chapter 6 Physical transformation of pure substances.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)07 Chapter 7 Simple mixtures.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)08 Chapter 8 Phase diagrams.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)09 Chapter 9 Chemical equilibrium.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)10 Chapter 10 Equilibrium electrochemistry.pdf
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)绪论 Tea Biochemistry(主讲:李大祥).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第一章 茶叶中的化学成分及其性质.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学试题库(无答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第二章 茶树次级代谢.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(试题).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第三章 环境对茶树物质代谢的作用.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(参考答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第四章 红茶制造化学 black tea processing chemistry、第五章 绿茶制造化学 第一节 绿茶制造中酶的热变性(1/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第五章 绿茶制造化学(2/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第六章 其他茶类及深加工化学.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十章 生物碱(Alkaloids).ppt
