《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Spectroscopy

SPECTROSCOPY Spectral Distribution of Radiant Energy Wave Number (cycles/cm) X-Ray UV Visible IR Microwave 200nm 400nm 800nm WAVELENGTH(nm)
SPECTROSCOPY Spectral Distribution of Radiant Energy Wave Number (cycles/cm) X-Ray UV Visible IR Microwave 200nm 400nm 800nm WAVELENGTH(nm)

V = Wave Number (cm-1) l = Wave Length C = Velocity of Radiation (constant) = 3 x 1010 cm/sec. u = Frequency of Radiation (cycles/sec) The energy of photon: h (Planck's constant) = 6.62 x 10-27 (Ergsec) V = u C l = E = h = h C l u C = l u C = ul SPECTROSCOPY
V = Wave Number (cm-1) l = Wave Length C = Velocity of Radiation (constant) = 3 x 1010 cm/sec. u = Frequency of Radiation (cycles/sec) The energy of photon: h (Planck's constant) = 6.62 x 10-27 (Ergsec) V = u C l = E = h = h C l u C = l u C = ul SPECTROSCOPY

SPECTRAL PROPERTIES,APPLICATIONS.AND INTERACTIONS OF ELECTROMAGNETIC RADIATION Electron kcsl/mol m Hz 0.4×107 4.1x10 33×1003×10" 10 9.4X10 4.1x1033×10d 3×10-9 10 X-ray 争 9.4×1034.1X102 3.3X10 3×10-7 10 9.4×1034.1×10°33X10 3×10 10 94×1014.1×10233×103x103 103 9.4×1g34.1×1g433×103x101 104 息4×104.1×1023×102 3×10 10 0.4X10r141x10033X104 3X103 107 Spectral properties,applications.and interactions of electromag- netic radiation

Spectral Properties.Applications.and Interactions of Electromagnetic Radiation atme' 33e wAa 4tte 争4e子4城 33490 ◆t014iap 1310 Micro 9102430°13e0 10

ELECTROMAGNETIC SPECTRUM wavelength,A,cm o 109 106 1021 10°1010° 食 1o2o 1018 10°10 1o510 02 frequency,cycles/sec visible region 400.500 600700 800 wavelength,X,my

DISPERSION OF POLYCHROMATIC LIGHT WITH A PRISM Polychromatic Ray Infrared Red Orange Yellow Green Blue Violet Ultraviolet monochromatic Ray SLIT PRISM Polychromatic Ray Monochromatic Ray Prism - spray out the spectrum and choose the certain wavelength (l) that you want by slit
DISPERSION OF POLYCHROMATIC LIGHT WITH A PRISM Polychromatic Ray Infrared Red Orange Yellow Green Blue Violet Ultraviolet monochromatic Ray SLIT PRISM Polychromatic Ray Monochromatic Ray Prism - spray out the spectrum and choose the certain wavelength (l) that you want by slit
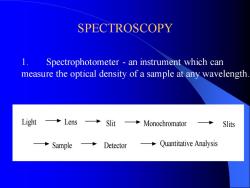
SPECTROSCOPY 1. Spectrophotometer - an instrument which can measure the optical density of a sample at any wavelength. Light Lens Slit Monochromator Sample Detector Quantitative Analysis Slits
SPECTROSCOPY 1. Spectrophotometer - an instrument which can measure the optical density of a sample at any wavelength. Light Lens Slit Monochromator Sample Detector Quantitative Analysis Slits

2. Fluorometer - measures the intensity of fluorescent light emitted by a sample exposed to UV light under specific conditions. Emit fluorescent light as energy decreases Ground state Sample 90C Detector UV Light Source Monochromator Monochromator Antibonding Antibonding Nonbonding Bonding Bonding Energy − − ' ' ' ' ' n-> n n->' Electron's molecular energy levels Fluorometer
2. Fluorometer - measures the intensity of fluorescent light emitted by a sample exposed to UV light under specific conditions. Emit fluorescent light as energy decreases Ground state Sample 90C Detector UV Light Source Monochromator Monochromator Antibonding Antibonding Nonbonding Bonding Bonding Energy − − ' ' ' ' ' n-> n n->' Electron's molecular energy levels Fluorometer
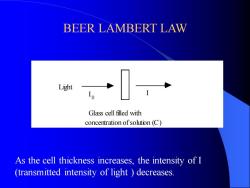
BEER LAMBERT LAW Glass cell filled with concentration of solution (C) I I Light 0 As the cell thickness increases, the intensity of I (transmitted intensity of light ) decreases
BEER LAMBERT LAW Glass cell filled with concentration of solution (C) I I Light 0 As the cell thickness increases, the intensity of I (transmitted intensity of light ) decreases
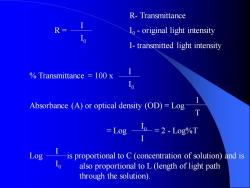
R- Transmittance R = I0 - original light intensity I- transmitted light intensity % Transmittance = 100 x Absorbance (A) or optical density (OD) = Log = Log = 2 - Log%T Log is proportional to C (concentration of solution) and is also proportional to L (length of light path through the solution). I I 0 I I 0 I 0 I 1 T I I 0
R- Transmittance R = I0 - original light intensity I- transmitted light intensity % Transmittance = 100 x Absorbance (A) or optical density (OD) = Log = Log = 2 - Log%T Log is proportional to C (concentration of solution) and is also proportional to L (length of light path through the solution). I I 0 I I 0 I 0 I 1 T I I 0
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Water.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Gas Liquid Chromatography.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Liquid Chromatography.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Protein.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Physical State of Ingredients in Food Systems.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Introduction.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Lipid.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Vitamin.ppt
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Enzyme Kinetics.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Primary sequence.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Protein Denaturation.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Modifications of Food Proteins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Protein Functionality.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Proteins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Chemical Kinetics.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Water activity and toxins.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Introduction.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Enzymes.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Carbohydrates.pdf
- 《食品化学 Food Chemistry》课程教学课件(讲稿,英文版)Effects of Lipid Oxidation.pdf
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Carbohydrate.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Minerals.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Lipids and Fats.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Water.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Experimental Foods.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Top Ten Food Companies Public and Private Companies.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Objective Evaluation of Food.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)General Food Safety.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Carbohydrates:Starch and Sugars.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Preservation.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Meat.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Milk and Milk Products.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Eggs.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Flour.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Enzymes.ppt
- 华东理工大学:《物理化学》课程教学资源(课件讲稿)绪论 Physical Chemistry.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)00 Chapter 0 Introduction.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)01 Chapter 1 The properties of gases.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)02 Chapter 2 The First Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)03 Chapter 3 The First Law the machinery.pdf
