华东理工大学:《物理化学》课程教学课件(讲稿,英文版)03 Chapter 3 The First Law the machinery

版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program
版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program

版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter we begin to unfold some of the power of thermodynamics by showing how to establish relations between different properties of a system. The procedure we use is based on the experimental fact that the internal energy and the enthalpy are state functions, and we derive a number of relations between observables by exploring the mathematical consequences of these facts. 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter we begin to unfold some of the power of thermodynamics by showing how to establish relations between different properties of a system. The procedure we use is based on the experimental fact that the internal energy and the enthalpy are state functions, and we derive a number of relations between observables by exploring the mathematical consequences of these facts. 3. The First Law: the machinery

版权所有:华东理工大学物理化学教研室 4 3.1 State functions 1). Exact and inexact differentials 2). Changes in internal energy 3). Expansion coefficient 3.2 The temperature dependence of the enthalpy 1). Changes in enthalpy at constant volume 2). The isothermal compressibility 3). The Joule -Thomson effect 3.3 The reaction between Cv and Cp 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 4 3.1 State functions 1). Exact and inexact differentials 2). Changes in internal energy 3). Expansion coefficient 3.2 The temperature dependence of the enthalpy 1). Changes in enthalpy at constant volume 2). The isothermal compressibility 3). The Joule -Thomson effect 3.3 The reaction between Cv and Cp 3. The First Law: the machinery
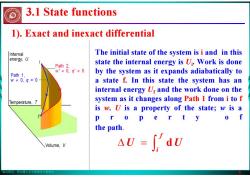
版权所有:华东理工大学物理化学教研室 5 The initial state of the system is i and in this state the internal energy is Ui. Work is done by the system as it expands adiabatically to a state f. In this state the system has an internal energy Uf and the work done on the system as it changes along Path 1 from i to f is w. U is a property of the state; w is a p r o p e r t y o f the path. 3.1 State functions 1). Exact and inexact differential ∫ Δ = f i U d U
版权所有:华东理工大学物理化学教研室 5 The initial state of the system is i and in this state the internal energy is Ui. Work is done by the system as it expands adiabatically to a state f. In this state the system has an internal energy Uf and the work done on the system as it changes along Path 1 from i to f is w. U is a property of the state; w is a p r o p e r t y o f the path. 3.1 State functions 1). Exact and inexact differential ∫ Δ = f i U d U

版权所有:华东理工大学物理化学教研室 6 In Path 2, the initial and final states are the same but in which the expansion is not adiabatic. In this path an energy q' enters the system as heat and the work w' is not the same as w . T h e w o r k a n d the heat are path functions. 1). Exact and inexact differential ∫ = f i q q ,path d 3.1 State functions
版权所有:华东理工大学物理化学教研室 6 In Path 2, the initial and final states are the same but in which the expansion is not adiabatic. In this path an energy q' enters the system as heat and the work w' is not the same as w . T h e w o r k a n d the heat are path functions. 1). Exact and inexact differential ∫ = f i q q ,path d 3.1 State functions
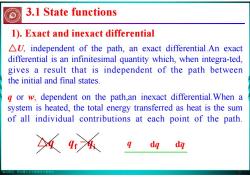
版权所有:华东理工大学物理化学教研室 7 △U, independent of the path, an exact differential.An exact differential is an infinitesimal quantity which, when integra-ted, gives a result that is independent of the path between the initial and final states. q or w, dependent on the path,an inexact differential.When a system is heated, the total energy transferred as heat is the sum of all individual contributions at each point of the path. 1). Exact and inexact differential △q qf - qi q dq dq 3.1 State functions
版权所有:华东理工大学物理化学教研室 7 △U, independent of the path, an exact differential.An exact differential is an infinitesimal quantity which, when integra-ted, gives a result that is independent of the path between the initial and final states. q or w, dependent on the path,an inexact differential.When a system is heated, the total energy transferred as heat is the sum of all individual contributions at each point of the path. 1). Exact and inexact differential △q qf - qi q dq dq 3.1 State functions

版权所有:华东理工大学物理化学教研室 8 Consider a perfect gas inside a cylinder fitted with a piston. Let the initial state be T, Vi and the final state be T, Vf . The change of state can be brought about in many ways, of which the two simplest are following: Path 1, in which there is free expansion against zero external pressure; Path 2, in which there is reversible, isothermal expansion. Calculate w , q , and Δ U f o r e a c h process. Example -Calculating work, heat, and internal energy
版权所有:华东理工大学物理化学教研室 8 Consider a perfect gas inside a cylinder fitted with a piston. Let the initial state be T, Vi and the final state be T, Vf . The change of state can be brought about in many ways, of which the two simplest are following: Path 1, in which there is free expansion against zero external pressure; Path 2, in which there is reversible, isothermal expansion. Calculate w , q , and Δ U f o r e a c h process. Example -Calculating work, heat, and internal energy

版权所有:华东理工大学物理化学教研室 9 Method: To find a starting point for a calculation in thermodynamics, it is often a good idea to go back to first principles, and to look for a way of expressing the quantity we are asked to calculate in terms of other quantities that are easier to calculate. Because the internal energy of a perfect gas arises only from the kinetic energy of its molecules, it is independent of volume; therefore, for any i s o t h e r m a l c h a n g e , i n general ΔU = q+w. Example -Calculating work, heat, and internal energy
版权所有:华东理工大学物理化学教研室 9 Method: To find a starting point for a calculation in thermodynamics, it is often a good idea to go back to first principles, and to look for a way of expressing the quantity we are asked to calculate in terms of other quantities that are easier to calculate. Because the internal energy of a perfect gas arises only from the kinetic energy of its molecules, it is independent of volume; therefore, for any i s o t h e r m a l c h a n g e , i n general ΔU = q+w. Example -Calculating work, heat, and internal energy

版权所有:华东理工大学物理化学教研室 10 Answer: Since ΔU = 0 for both paths and ΔU = q+w. The work of free expansion is zero, in Path 1, w = 0 and q = 0; for Path 2, the work is given by so w = - nRT ln (Vf /Vi), and q = nRT ln (Vf /Vi). i f ln f d i V V nRT V V w nRT V V = − = − ∫ Example -Calculating work, heat, and internal energy
版权所有:华东理工大学物理化学教研室 10 Answer: Since ΔU = 0 for both paths and ΔU = q+w. The work of free expansion is zero, in Path 1, w = 0 and q = 0; for Path 2, the work is given by so w = - nRT ln (Vf /Vi), and q = nRT ln (Vf /Vi). i f ln f d i V V nRT V V w nRT V V = − = − ∫ Example -Calculating work, heat, and internal energy
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)02 Chapter 2 The First Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)01 Chapter 1 The properties of gases.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)00 Chapter 0 Introduction.pdf
- 华东理工大学:《物理化学》课程教学资源(课件讲稿)绪论 Physical Chemistry.pdf
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Enzymes.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Flour.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Eggs.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Milk and Milk Products.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Meat.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Preservation.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Carbohydrates:Starch and Sugars.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)General Food Safety.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Objective Evaluation of Food.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Top Ten Food Companies Public and Private Companies.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Experimental Foods.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Water.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Lipids and Fats.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Minerals.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Carbohydrate.ppt
- 《食品化学与分析 Food Chemistry and Analysis》课程PPT教学课件(英文版)Spectroscopy.ppt
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)04 br Chapter 4 The Second Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)05 Chapter 5 The Second Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)06 Chapter 6 Physical transformation of pure substances.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)07 Chapter 7 Simple mixtures.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)08 Chapter 8 Phase diagrams.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)09 Chapter 9 Chemical equilibrium.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)10 Chapter 10 Equilibrium electrochemistry.pdf
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)绪论 Tea Biochemistry(主讲:李大祥).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第一章 茶叶中的化学成分及其性质.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学试题库(无答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第二章 茶树次级代谢.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(试题).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第三章 环境对茶树物质代谢的作用.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(参考答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第四章 红茶制造化学 black tea processing chemistry、第五章 绿茶制造化学 第一节 绿茶制造中酶的热变性(1/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第五章 绿茶制造化学(2/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第六章 其他茶类及深加工化学.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十章 生物碱(Alkaloids).ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十一章 鞣质.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十二章 其他成分.ppt
