华东理工大学:《物理化学》课程教学课件(讲稿,英文版)07 Chapter 7 Simple mixtures

版权所有:华东理工大学物理化学教研室 上一页 下一页 返回目录 Part 1: Equilibrium 7. Simple mixtures
版权所有:华东理工大学物理化学教研室 上一页 下一页 返回目录 Part 1: Equilibrium 7. Simple mixtures

版权所有:华东理工大学物理化学教研室 2 The chapter begins by developing the concept of chemical potential to show that it is a particular of a class of partial molar quantities. Then it explores how the chemical potential of a substance is used to describe the physical properties of mixture. The underlying principle is that at equilibrium the chemical potential of a species is the same in every phase. 7. Simple mixtures
版权所有:华东理工大学物理化学教研室 2 The chapter begins by developing the concept of chemical potential to show that it is a particular of a class of partial molar quantities. Then it explores how the chemical potential of a substance is used to describe the physical properties of mixture. The underlying principle is that at equilibrium the chemical potential of a species is the same in every phase. 7. Simple mixtures

版权所有:华东理工大学物理化学教研室 3 The thermodynamic description of mixtures 7.1 Partial molar quantities 7.2 The thermodynamics of mixing 7.3 The chemical potentials of liquid The properties of solutions 7.4 Liquid mixtures 7.5 Colligative properties Activities 7.6 The solvent activity 7.7 The solute activity 7. Simple mixtures
版权所有:华东理工大学物理化学教研室 3 The thermodynamic description of mixtures 7.1 Partial molar quantities 7.2 The thermodynamics of mixing 7.3 The chemical potentials of liquid The properties of solutions 7.4 Liquid mixtures 7.5 Colligative properties Activities 7.6 The solvent activity 7.7 The solute activity 7. Simple mixtures
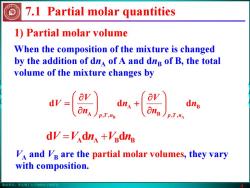
版权所有:华东理工大学物理化学教研室 4 1) Partial molar volume When the composition of the mixture is changed by the addition of dnA of A and dnB of B, the total volume of the mixture changes by 7.1 Partial molar quantities B B , A A , d d d B A n n V n n V V nTp nTp ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = = + ddd nVnVV BBAA VA and VB are the partial molar volumes, they vary with composition
版权所有:华东理工大学物理化学教研室 4 1) Partial molar volume When the composition of the mixture is changed by the addition of dnA of A and dnB of B, the total volume of the mixture changes by 7.1 Partial molar quantities B B , A A , d d d B A n n V n n V V nTp nTp ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = = + ddd nVnVV BBAA VA and VB are the partial molar volumes, they vary with composition

版权所有:华东理工大学物理化学教研室 5 Once the partial molar volumes of the two components of a mixture at the composition (and T) of interest are known, we can state the total volume, V, of the mixture by using = + VnVnV BBAA 7.1 Partial molar quantities
版权所有:华东理工大学物理化学教研室 5 Once the partial molar volumes of the two components of a mixture at the composition (and T) of interest are known, we can state the total volume, V, of the mixture by using = + VnVnV BBAA 7.1 Partial molar quantities

版权所有:华东理工大学物理化学教研室 6 • The determination of partial molar volume 7.1 Partial molar quantities With particular values of the parameters A, B, and C, then the partial molar volume of A at any composition could be obtained from A A , A 2CnB n V V nTp B += ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J ', J nTp n V V ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
版权所有:华东理工大学物理化学教研室 6 • The determination of partial molar volume 7.1 Partial molar quantities With particular values of the parameters A, B, and C, then the partial molar volume of A at any composition could be obtained from A A , A 2CnB n V V nTp B += ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J ', J nTp n V V ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =

版权所有:华东理工大学物理化学教研室 7 The determination of partial molar volume 7.1 Partial molar quantities B 2 A B AA B )1( n CnA n VnV V +− = − = The partial molar volume of the second component is obtained from = + VnVnV BBAA
版权所有:华东理工大学物理化学教研室 7 The determination of partial molar volume 7.1 Partial molar quantities B 2 A B AA B )1( n CnA n VnV V +− = − = The partial molar volume of the second component is obtained from = + VnVnV BBAA

版权所有:华东理工大学物理化学教研室 8 7.1 Partial molar quantities The molar volume: the volume that 1 mol substance occupies, which is always positive. The partial molar volume: the contribution of 1 mol of a component to the volume of the mixture at a specific T and p, which may be positive or negative. The determination of partial molar volume
版权所有:华东理工大学物理化学教研室 8 7.1 Partial molar quantities The molar volume: the volume that 1 mol substance occupies, which is always positive. The partial molar volume: the contribution of 1 mol of a component to the volume of the mixture at a specific T and p, which may be positive or negative. The determination of partial molar volume

版权所有:华东理工大学物理化学教研室 9 Partial molar quantities The concept of a partial molar quantity can be extended to any extensive state function. Let X denote any extensive properties of a system that contains K components 7.1 Partial molar quantities The change in X When dT, dp, dni ) ,( 21 nnnpTXX K = ⋅⋅⋅ ∑ = ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ ⎟ + ⎠⎞ ⎜⎝⎛ ∂∂ = Ki np nT npT n nX p pX T TX X ij 1 i , i , , d d d d j j
版权所有:华东理工大学物理化学教研室 9 Partial molar quantities The concept of a partial molar quantity can be extended to any extensive state function. Let X denote any extensive properties of a system that contains K components 7.1 Partial molar quantities The change in X When dT, dp, dni ) ,( 21 nnnpTXX K = ⋅⋅⋅ ∑ = ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ ⎟ + ⎠⎞ ⎜⎝⎛ ∂∂ = Ki np nT npT n nX p pX T TX X ij 1 i , i , , d d d d j j

版权所有:华东理工大学物理化学教研室 10 7.1 Partial molar quantities Definition npT ij i i n X X ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = , def X and ni are extensive properties, and they are independent of the total quantities in systems; however, the partial molar quantities, Xi , is intensive property. ∑ = =+⋅⋅⋅+= K 1 2211 KK i XnXnXnXnX ii Partial molar quantities
版权所有:华东理工大学物理化学教研室 10 7.1 Partial molar quantities Definition npT ij i i n X X ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = , def X and ni are extensive properties, and they are independent of the total quantities in systems; however, the partial molar quantities, Xi , is intensive property. ∑ = =+⋅⋅⋅+= K 1 2211 KK i XnXnXnXnX ii Partial molar quantities
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)06 Chapter 6 Physical transformation of pure substances.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)05 Chapter 5 The Second Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)04 br Chapter 4 The Second Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)03 Chapter 3 The First Law the machinery.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)02 Chapter 2 The First Law the concepts.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)01 Chapter 1 The properties of gases.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)00 Chapter 0 Introduction.pdf
- 华东理工大学:《物理化学》课程教学资源(课件讲稿)绪论 Physical Chemistry.pdf
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Enzymes.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Flour.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Eggs.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Milk and Milk Products.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Meat.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Food Preservation.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Carbohydrates:Starch and Sugars.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)General Food Safety.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Objective Evaluation of Food.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Top Ten Food Companies Public and Private Companies.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Experimental Foods.ppt
- 《Food Safety and Home Food Preservation》课程教学课件(PPT讲稿)Water.ppt
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)08 Chapter 8 Phase diagrams.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)09 Chapter 9 Chemical equilibrium.pdf
- 华东理工大学:《物理化学》课程教学课件(讲稿,英文版)10 Chapter 10 Equilibrium electrochemistry.pdf
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)绪论 Tea Biochemistry(主讲:李大祥).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第一章 茶叶中的化学成分及其性质.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学试题库(无答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第二章 茶树次级代谢.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(试题).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第三章 环境对茶树物质代谢的作用.ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(试卷习题)茶叶生物化学期末考试A卷(参考答案).doc
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第四章 红茶制造化学 black tea processing chemistry、第五章 绿茶制造化学 第一节 绿茶制造中酶的热变性(1/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第五章 绿茶制造化学(2/2).ppt
- 安徽农业大学:《茶叶生物化学》课程教学资源(PPT课件)第六章 其他茶类及深加工化学.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十章 生物碱(Alkaloids).ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十一章 鞣质.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十二章 其他成分.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第十三章 中药复方药效物质基础研究.ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(试卷习题)中药化学试题.doc
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第一章 绪论(主讲:王栋、关枫).ppt
- 黑龙江中医药大学:《中药化学》课程教学资源(PPT课件)第二章 中药化学成分的一般研究方法.ppt
