北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 18 Hydrogen and the rare gases

18 Hydrogen and the rare gases 8.1 The discovery of the rare gases 18.2 Properties and uses of the rare gases -18.3 The existence and recovery of the rare gases -18.4 The rare gas compounds -18.5 Summarry on the main group elements
18.1 The discovery of the rare gases §18 Hydrogen and the rare gases 18.4 The rare gas compounds 18.3 The existence and recovery of the rare gases 18.2 Properties and uses of the rare gases 18.5 Summarry on the main group elements

18.1 The discovery of the rare gases Rare gases:He Ne Ar Kr Xe Rn Valence e:ns2npo “第三位小数的胜利” fractionate N2 from air:1.2572 gL Ar Chemical preparation of N2:1.2505gL-1
18.1 The discovery of the rare gases Ar Rare gases:He Ne Ar Kr Xe Rn “第三位小数的胜利” fractionate N2 from air:1.2572 g•L-1 Chemical preparation of N2:1.2505g•L-1 Valence e-:ns 2np 6
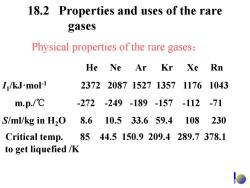
18.2 Properties and uses of the rare gases Physical properties of the rare gases: He Ne Ar Kr Xe Rn I/kJ-mol-1 237220871527135711761043 m.p./℃ -272-249-189-157-112 -71 S/ml/kg in H2O 8.6 10.533.659.4 108 230 Critical temp. 8544.5150.9209.4289.7378.1 to get liquefied /K
18.2 Properties and uses of the rare gases He Ne Ar Kr Xe Rn I1 /kJ·mol-1 2372 2087 1527 1357 1176 1043 m.p./℃ -272 -249 -189 -157 -112 -71 S/ml/kg in H2O 8.6 10.5 33.6 59.4 108 230 Physical properties of the rare gases: to get liquefied /K Critical temp. 85 44.5 150.9 209.4 289.7 378.1

18.4 The rare gas compounds 1.Synthesis:XePtF(red crystal) idea思路:O2[PtF6]has been synthesized 02→0+eL1=1171.5 kJ.mol Xe→Xe+e 11=1176.5kJ.mol 'o=201pm 138940Z,Z41-〉 rxe =210pm R prediction预测:Xe+PtF。→Xe[PtE。]
18.4 The rare gas compounds 1.Synthesis:XePtF6 (red crystal) idea 思路:O2 [PtF6 ] has been synthesized Xe PtF Xe[PtF ] 预测: 6 6 ) 1 (1 138940 0 1 2 R n Z Z A U r Xe 210pm 201pm O2 r -1 Xe Xe e 1 1176.5kJmol I -1 O2 O2 e 1 1171.5kJmol I prediction
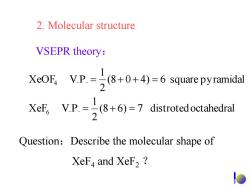
2.Molecular structure VSEPR theory: XeOF,V.P.-(8+0+4)-6 squarepyramidal 2 XeF,V.P.)7distroted octahedral Question:Describe the molecular shape of XeF,and XeF2
2. Molecular structure VSEPR theory: (8 6) 7 distrotedoctahedral 2 1 XeF V.P. (8 0 4) 6 square pyramidal 2 1 XeOF V.P. 6 4 Question:Describe the molecular shape of XeF4 and XeF2 ?

18.5 Summarry on the main group elements *18.5.1 The general properties of the s-block elements 18.5.2 Outline on the p-block elements 18.5.3 The property variations for compounds of p-block elements
§ 18.5 Summarry on the main group elements *18.5.1 The general properties of the s-block elements 18.5.2 Outline on the p-block elements 18.5.3 The property variations for compounds of p-block elements

*18.5.1 The general properties of the s-block elements The alkali metals (IA):ns Li,Na,K,Rb,Cs,Fr The alkaline metals(IIA):ns2 Be,Mg,Ca,Sr,Ba,Ra They are all active metals D
*18.5.1 The general properties of the s-block elements The alkali metals (IA ):ns1 Li, Na, K, Rb, Cs, Fr The alkaline metals (IIA ):ns2 Be, Mg, Ca, Sr, Ba, Ra They are all active metals
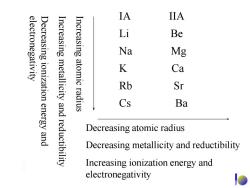
IA IIA electronegativity Decreasing ionization energy and Increasing metallicity Increasing atomic Li Be Na Mg K Ca Rb Sr and radius Cs Ba reductibility Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity
electronegativity Decreasing ionization energy and Increasing metallicity and reductibility Increasing atomic radius IA IIA Li Be Na Mg K Ca Rb Sr Cs Ba Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity
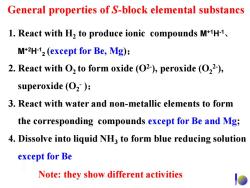
General properties of S-block elemental substancs 1.React with H2 to produce ionic compounds M+1H-1. M+2H-12(except for Be,Mg); 2.React with O2 to form oxide (O2),peroxide (O22), superoxide (O2); 3.React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4.Dissolve into liquid NH3 to form blue reducing solution except for Be Note:they show different activities
1. React with H2 to produce ionic compounds M+1H-1 、 M+2H-1 2 (except for Be, Mg); 2. React with O2 to form oxide (O2- ), peroxide (O2 2- ), superoxide (O2 - ); 3. React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4. Dissolve into liquid NH3 to form blue reducing solution except for Be General properties of S-block elemental substancs Note: they show different activities

18.5.2 Outline on the p-block elements 鳎 1 18 IA 13 1415 16 17 0 H IIIA IVA VA VIA VIA He Li Be B 0 F Ne 3 5 6 7 8 9 1011 12 Na g IIIB IVB VB VIB VIB 四 IB IIB Al Si P c1 Ax K Ca Se Ti Cr n Fe Co Ni Cu Zn Ga Ge As Se Bx Kr Rb Y Zx b Mo Tc Ru R贴h Pd Ag Cd In Sn Sb Te Xe Cs Ba Lu Ha Ta Re 0s Pt Au Pb Bi Po At Rn Ra Lx Rf Db Sg Bh Hs Mt 调系 Px Nb Sm Eu Gd Tb Dy Ho Ex Tm Yb 铜系 Th Pa Pu An Cm Bk cEsEm Md No
18.5.2 Outline on the p-block elements
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 17 The halogens.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 16 The elements of oxygen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 15 The elements of nitrogen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 14 The elements of carbon family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 13 The elements of boron family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 12 The alkali and alkaline earth metal.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 11 Basic of Coordination Chemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 10 Reduction - oxidization Reactions.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 9 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 8 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 7 Crystal Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 6 Molecular Structure and covalent bond theory.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 5 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 4 Chemical equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 2 Basic of thermodynamics.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 1 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 9 Molecular Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 8 Atomic Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 7 Redox Reactions and the Base of Electrochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 16 The d-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 19 The elements of copper and zinc family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 21 The elements of chromium and Manganese family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 22 The elements of iron and platinium families.pdf
- 广东医科大学:《有机化学》课程教学资源(教学大纲)有机化学教学大纲(药学专业)Organic Chemistry.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第一章 绪论 Organic Chemistry(负责人:杨雪梅).pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第三章 烯烃 Alkenes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第二章 烷烃和环烷烃 Alkane and Cycloalkane.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第四章 炔烃和二烯烃 Alkynes and Dienes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第五章 二烯烃 CnH2n-2.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第六章 立体化学基础.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第七章 芳香烃.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第九章 醛和酮 aldehyde and ketone.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十章 羧酸和取代羧酸.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十二章 羧酸衍生物 Carboxylic acid derivatives.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十三章 碳负离子的反应.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十五章 杂环化合物.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第七章 卤代烃.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第八章 醇、酚和醚.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十五章 糖类 saccharide.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十四章 有机含氮化合物.pdf
