北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 13 The elements of boron family

Chapter 13 The elements of boron family 13.1 Outline on boron family 13.2 The elemental substances of boron family 13.3 The compounds of boron -*13.4 The compounds of aluminum
Chapter 13 The elements of boron family *13.4 The compounds of aluminum 13.3 The compounds of boron 13.2 The elemental substances of boron family 13.1 Outline on boron family

13.1 Outline on boron family Boron family (IA):B,Al,Ga,In,TI Valence electron configuration:ns2npl Electron deficient elements:number of valence electrons number of valence orbits Electron deficient compounds:number of electron pairs in bonding molecular orbits number of valence orbits(即:还有空的成键轨道) Example:BF3(sp3),H3BO3.B:2S22pl HBF is not electron deficient compound
13.1 Outline on boron family Boron family (ⅢA):B,Al,Ga,In,Tl Valence electron configuration:ns 2np 1 Electron deficient elements: number of valence electrons < number of valence orbits Electron deficient compounds: number of electron pairs in bonding molecular orbits < number of valence orbits(即:还有空的成键轨道). Example:BF3 (sp3 ), H3BO3。 B: 2S22p1 HBF4 is not electron deficient compound

21p 2P 2s1 excited 2s↑↑)( $p2 B:2s22p1 sp-hybridization The formation of BF
of BF3 The formation 2s 2p 2p 2s sp 2 sp2 hybridization B : 2s 22p 1 excited
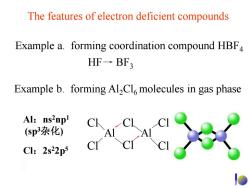
The features of electron deficient compounds Example a.forming coordination compound HBF4 HF→BF3 Example b.forming Al2Cl6 molecules in gas phase Al:ns2npl C (sp3杂化) Cl:2s22p5
Cl Cl Al Cl Cl Cl Cl Al The features of electron deficient compounds Example b. forming Al2Cl6 molecules in gas phase HF BF3 Example a. forming coordination compound HBF4 Al:ns2np1 (sp3杂化) Cl:2s22p5

General properties of boron family elements Except for B which is a metalloid(非金属),Al, Ga,In,Tl are all metals. Oxidation numbers:B,Al,Ga:(+3) In:(+1,+3) T:(+1) Maximum coordination numbers: B:4 example:HBF4(sp3杂化) others:6 example:Na3AlF6(sp3d2杂化)
General properties of boron family elements • Except for B which is a metalloid(非金属), Al, Ga,In,Tl are all metals. • Oxidation numbers:B,Al,Ga:(+3) In:(+1,+3) Tl:(+1) • Maximum coordination numbers: B:4 example:HBF4 (sp3杂化) others:6 example:Na3AlF6 (sp3d 2杂化)

13.2 The elemental substances of boron family 硼B metalloid 铝Al meta 镓Ga metal 铟In metal
13.2 The elemental substances of boron family metalloid metal metal metal

The elemental substances of boron (denote B) Allotropes(同素异形体):amorphous B-→brown powder (a few types)crystalline B->black-gray Properties:high chemical activity,high hardness high m.p and b.p a一菱形硼(B12)(one of the crystalline type of B): Atomic crystal 12 vertices,20 faces
The elemental substances of boron (denote B) Allotropes(同素异形体):amorphous B brown powder (a few types) crystalline B black-gray Properties: high chemical activity, high hardness high m.p and b.p α -菱形硼 (B12) (one of the crystalline type of B): Atomic crystal 12 vertices, 20 faces
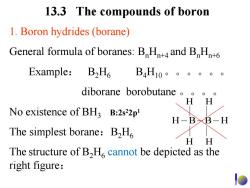
13.3 The compounds of boron 1.Boron hydrides(borane) General formula of boranes:B Hn+4 and BHn+6 Example:B2H6 B4H10。。。。。o diborane borobutane H H No existence of BH B:2s22p1 H-BB-H The simplest borane:B2H HH The structure of B,H cannot be depicted as the right figure:
13.3 The compounds of boron 1. Boron hydrides (borane) General formula of boranes: BnHn+4 and BnHn+6 Example: B2H6 B4H10。。。。。。 diborane borobutane 。。。。 No existence of BH3 The simplest borane:B2H6 The structure of B2H6 cannot be depicted as the right figure: H H B B H H H H B:2s22p1
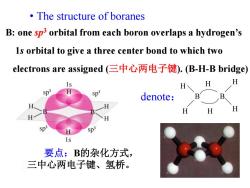
The structure of boranes B:one sp3 orbital from each boron overlaps a hydrogen's 1s orbital to give a three center bond to which two electrons are assigned(三中心两电子键).(B-H-B bridge) H H H Sp3 p denote: B B H SD 1s 要点:B的杂化方式, 三中心两电子键、氢桥
• The structure of boranes B: one sp3 orbital from each boron overlaps a hydrogen’s 1s orbital to give a three center bond to which two electrons are assigned (三中心两电子键). (B-H-B bridge) denote: H H B B H H H H 要点:B的杂化方式, 三中心两电子键、氢桥
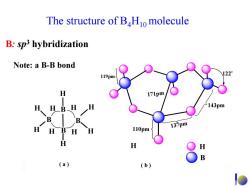
The structure of BHio molecule B:sp3 hybridization Note:a B-B bond 119pm H 143pm 13 H H 110pm B (a)) (b)
The structure of B4H10 molecule 119pm 110pm 137pm 143pm 122。 171pm H B ( b ) B B B B H H H H H H H H H H H ( a ) B: sp3 hybridization Note: a B-B bond
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 12 The alkali and alkaline earth metal.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 11 Basic of Coordination Chemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 10 Reduction - oxidization Reactions.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 9 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 8 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 7 Crystal Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 6 Molecular Structure and covalent bond theory.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 5 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 4 Chemical equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 2 Basic of thermodynamics.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 1 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 9 Molecular Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 8 Atomic Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 7 Redox Reactions and the Base of Electrochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 16 The d-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 15 The p-block elements(Ⅲ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 14 Chapter 14 The p-block elements(Ⅱ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 13 The p-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 12 The s-Block Elements.pptx
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 11 Coordination Compound Structures.ppt
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 14 The elements of carbon family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 15 The elements of nitrogen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 16 The elements of oxygen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 17 The halogens.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 18 Hydrogen and the rare gases.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 19 The elements of copper and zinc family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 21 The elements of chromium and Manganese family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 22 The elements of iron and platinium families.pdf
- 广东医科大学:《有机化学》课程教学资源(教学大纲)有机化学教学大纲(药学专业)Organic Chemistry.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第一章 绪论 Organic Chemistry(负责人:杨雪梅).pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第三章 烯烃 Alkenes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第二章 烷烃和环烷烃 Alkane and Cycloalkane.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第四章 炔烃和二烯烃 Alkynes and Dienes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第五章 二烯烃 CnH2n-2.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第六章 立体化学基础.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第七章 芳香烃.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第九章 醛和酮 aldehyde and ketone.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十章 羧酸和取代羧酸.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十二章 羧酸衍生物 Carboxylic acid derivatives.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十三章 碳负离子的反应.pdf
