北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 4 Chemical equilibria

Chapter 4Chemical equilibria 4.1 The state of a chemical reaction at equilibrium X 4.2 Direction of a chemical reaction 4.3 The relationship between standard equilibrium constant Ke and A,G X 4.4 The shift of a chemical equilibrium
Chapter 4 Chemical equilibria §4.3 The relationship between standard equilibrium constant K and rGm §4.2 Direction of a chemical reaction §4.1 The state of a chemical reaction at equilibrium §4.4 The shift of a chemical equilibrium

4.1 The state of a chemical reaction at equilibrium 4.1.1.The basic features of a chemical equilibrium Most reactions are reversible,an example is molH(x1x1 mol.L.s- t/s 0 0.01000.0100 0 7.60 0 2000 0.003970.003970.0121 1.20 2.04 4850 0.002130.002130.0157 0.3453.43 85000.002130.002130.01570.345 3.43 多
0 0.0100 0.0100 0 7.60 0 2000 0.00397 0.00397 0.0121 1.20 2.04 4850 0.00213 0.00213 0.0157 0.345 3.43 Most reactions are reversible, an example is t/s 1 /mol L c 6 10 7 10 1 1 mol L s H (g) I (g) 2HI(g) 2 2 8500 0.00213 0.00213 0.0157 0.345 3.43 §4.1 The state of a chemical reaction at equilibrium 4.1.1. The basic features of a chemical equilibrium

Initially,concentrations of H,and I,are large,only the forward reaction occurs;As time passes,the concentrations of H,and I, decrease and in result the forward reaction slows down.The concentrations of HI increase and in result the reverse reaction speeds up. The reaction mixture is at equilibrium until the forward and reverse reactions go at the same rate
Initially, concentrations of H2 and I2 are large, only the forward reaction occurs; As time passes, the concentrations of H2 and I2 decrease and in result the forward reaction slows down. The concentrations of HI increase and in result the reverse reaction speeds up. The reaction mixture is at equilibrium until the forward and reverse reactions go at the same rate

(57-1ow)ia I-Tlow/ HI )通 H2. t/s t/s H2(g)+I,(g)=2HI(g) HI 2HI(g))≠H2(g)+2(g) H2,k t/s
正 逆 正 逆 H (g) I (g) 2HI(g) 2 2 2HI(g) H2 (g) + I2 (g) Conc
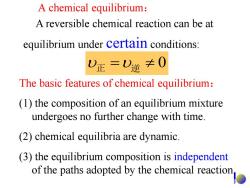
A chemical equilibrium: A reversible chemical reaction can be at equilibrium under certain conditions: 0正=)逆≠0 The basic features of chemical equilibrium: (1)the composition of an equilibrium mixture undergoes no further change with time. (2)chemical equilibria are dynamic. (3)the equilibrium composition is independent of the paths adopted by the chemical reaction
A chemical equilibrium: 正 逆 0 A reversible chemical reaction can be at equilibrium under certain conditions: The basic features of chemical equilibrium: (1) the composition of an equilibrium mixture undergoes no further change with time. (2) chemical equilibria are dynamic. (3) the equilibrium composition is independent of the paths adopted by the chemical reaction
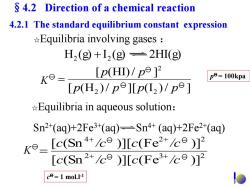
4.2 Direction of a chemical reaction 4.2.1 The standard equilibrium constant expression *Equilibria involving gases H2 (g+12(g=2HI(g Ke- [p(H)/p9]2 p=100kpa p(H2)/p9]Lp(L2)/pe] Equilibria in aqueous solution: Sn2+(aq)+2Fe3+(aq)-Sn4+(aq)+2Fe2(aq) ko_[e(Sn)]Ic(Fe2+/ce) [c(Sn2ce )]c(Fe+ce)12 c=1 mol.I-1
☆Equilibria in aqueous solution: H (g) I (g) 2HI(g) 2 2 ☆Equilibria involving gases : Sn2+(aq)+2Fe3+(aq) Sn4+ (aq)+2Fe2+(aq) [ (H )/ ][ (I )/ ] [ (HI)/ ] 2 2 2 p p p p p p K p = 100kpa 2 3 2 4 2 2 [ (Sn )][ (Fe )] [ (Sn )][ (Fe )] c /c c /c c /c c /c K c = 1 mol.l-1 4.2.1 The standard equilibrium constant expression §4.2 Direction of a chemical reaction

For a more general chemical reaction: aA(g)+bB(aq)+cC(s)=xX(g)+yY(aq)+zZ(1) k3=LpK/ppy/c9Ψ p=100kpa Ip(A)/pe]alc(B)Ic c=1 mol.I-1 *The value of a constant ke depends on temperature and is independent of concen- trations and partial pressures of the reaction components..Kis a dimensionless quantity(无量纲,google) *The form of a standard equilibrium constant expression depends exactly on how a stoichio- metric chemical equation is written
For a more general chemical reaction: aA(g) bB(aq) cC(s) xX(g) yY(aq) zZ(l) K b c c a p p y c c x p p A / B / X / Y / The value of a constant depends on temperature and is independent of concen- trations and partial pressures of the reaction components. K The form of a standard equilibrium constant expression depends exactly on how a stoichio- metric chemical equation is written. K is a dimensionless quantity (无量纲,google) p = 100kpa c = 1 mol.l-1

H2(g+,(g=2HI(g) [p(H)/p912 [p(H)/pe]p(I,)/pe] H2(g+号12(g)一Hg) 2 月 2 K- [P(HD)/pe] p(H)( 2HΠ(g)—H2(g)+I2(g) I(H)1-( [p(H)/pe]2
H (g) I (g) 2HI(g) 2 2 [ (H )/ ][ (I )/ ] [ (HI)/ ] 2 2 2 p p p p p p 2 I 2 (g) HI(g) 2 1 H (g) 2 1 ( )1/2 1/ 2 2 1/ 2 2 [ (H )/ ] [ (I )/ ] [ (HI)/ ] p p p p p p 2HI(g) H (g) I (g) 2 2 =( )-1 [ (H ) / ][ (I ) / ] [ (HI) / ] 2 2 2 p p p p p p K1 K1 K3 K3 K1 K2 K1 K2

The principle of multiple equilibria Example:Equilibrium constants for the following reactions have been determined: ①BrC1(g)=1/2Cl2(g)+1/2Br2(g)K,o=0.67 ②L2(g)+Br2(g)=2IBr(g)K8=0.051 Using the above information,calculate the standard equilibrium constant for the following reaction: 32BrCI (g)+12(g)-2IBr(g)+Cl2(g) Answer:①x2+②=③ 2BrCl (g)+12(g)=2IBr(g)+Cl2(g) K月=()29=0.672X0.051=0.023
Example:Equilibrium constants for the following reactions have been determined: The principle of multiple equilibria Answer:①x2 + ② = ③ K 2 ②I2 (g)+Br2 (g) 2IBr(g) = 0.051 Using the above information, calculate the standard equilibrium constant for the following reaction: ①BrCl(g) 1/2Cl2 (g)+1/2Br2 (g) K1 = 0.67 2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) ③2BrCl (g)+ I2 (g) 2IBr(g)+ Cl2 (g) K3 = · = 0.67 K3 K1 K2 2×0.051=0.023 2 ( )

The principle of multiple equilibria K月=(K2k9=0.672×0.051=0.023 Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations,the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions'reaction equilibrium constant. ------a principle of multi-equilibria 结论:如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商—多重平衡原理
The principle of multiple equilibria Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations, the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions’ reaction equilibrium constant. ------a principle of multi-equilibria 结论: 如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商多重平衡原理. = · = 0.67 K3 K 2×0.051=0.023 1 K2 2 ( )
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 2 Basic of thermodynamics.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 1 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 9 Molecular Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 8 Atomic Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 7 Redox Reactions and the Base of Electrochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 16 The d-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 15 The p-block elements(Ⅲ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 14 Chapter 14 The p-block elements(Ⅱ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 13 The p-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 12 The s-Block Elements.pptx
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 11 Coordination Compound Structures.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 10 Solid Structure.pptx
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 4 Chemical equilibria, entropy and Gibbs function.pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 4 Chemical equilibria, entropy and Gibbs function.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 6 Precipitation-Solubility Equilibria.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 5 Acid-Base Equilibrium.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 2 Thermochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 1 Preface.ppt
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 8 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 7 Redox Reactions and the Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 5 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 6 Molecular Structure and covalent bond theory.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 7 Crystal Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 8 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 9 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 10 Reduction - oxidization Reactions.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 11 Basic of Coordination Chemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 12 The alkali and alkaline earth metal.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 13 The elements of boron family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 14 The elements of carbon family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 15 The elements of nitrogen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 16 The elements of oxygen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 17 The halogens.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 18 Hydrogen and the rare gases.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 19 The elements of copper and zinc family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 21 The elements of chromium and Manganese family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 22 The elements of iron and platinium families.pdf
- 广东医科大学:《有机化学》课程教学资源(教学大纲)有机化学教学大纲(药学专业)Organic Chemistry.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第一章 绪论 Organic Chemistry(负责人:杨雪梅).pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第三章 烯烃 Alkenes.pdf
