北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 12 The alkali and alkaline earth metal

Chapter 12 The alkali and alkaline earth meta X 12.1 The elementary substances 12.2 The properties of the alkali and alkaline earth compounds X$12.3 The particularity of Li and Be The diagonal relationship
§12.3 The particularity of Li and Be The diagonal relationship §12.2 The properties of the alkali and alkaline earth compounds §12.1 The elementary substances Chapter 12 The alkali and alkaline earth metal

12.1 The elementary substances 12.1.1 Properties of the elementary substances 1.Physical properties ☆Metallic luster; ☆Low densities;. ☆Soft; ☆Low melting point;: *Good conductor of heat and electricity Na Li K
§12.1.1 Properties of the elementary substances Na Li K 1.Physical properties §12.1 The elementary substances ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; ☆Good conductor of heat and electricity

Rb 181 Low melting point Be Mg Ca 98 6 29 Sr Ba Li Na K Rb Cs Fr
Be Mg Ca Sr Ba Rb Cs Low melting point Li Na K Rb Cs Fr

2.Chemical properties: React with oxygen,sulfur,nitrogen and halogen to form their corresponding compounds. The elementary substances form the corresponding oxides when they burn in air: Li2O Na202 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 Na,O, Pale yellow Pale yellow Magnesium burning in air
The elementary substances form the corresponding oxides when they burn in air: Li2O Na2O2 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 • React with oxygen, sulfur, nitrogen and halogen to form their corresponding compounds. 2.Chemical properties: Magnesium burning in air Li2O Na2O2 KO2 Pale yellow Pale yellow
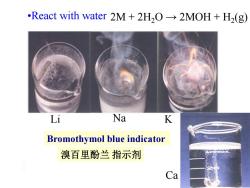
.React with water 2M+2H,O->2MOH+H2(g) Li Na K Bromothymol blue indicator 溴百里酚兰指示剂 Ca
•React with water Li Na K Ca 2M + 2H2O → 2MOH + H2 (g) Bromothymol blue indicator 溴百里酚兰 指示剂

React with liquid ammonia: M(S+(c+y)NH3①= M(NH3)x+e(NH3) (Alkali metal and Ca,Sr,Ba) 导电性,顺磁性 a blue solution 痕量杂质, 2M(s)+2NH3(I)2M++2NH2+H2(g) 或光化作用 cfM+H20→Mt+OH+H2(g)
•React with liquid ammonia: a blue solution 2M(s) + 2NH3 (l) 2M+ + 2NH2 - + H2 (g) c.f. M + H2O M+ + OH- + H2 (g) M(s) + (x + y )NH3 (l) M(NH3 )x + + e(NH3 )y - 导电性,顺磁性 痕量杂质, 或光化作用 (Alkali metal and Ca, Sr, Ba)
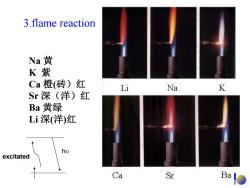
3.flame reaction Na黄 K紫 Ca橙(砖)红 Li Na K Sr深(洋)红 Ba黄绿 Li深(洋)红 excitated Ca Sr Ba
3.flame reaction Na 黄 K 紫 Ca 橙(砖)红 Sr 深(洋)红 Ba 黄绿 Li 深(洋)红 excitated h

S 12.1.2 The existence and preparation of s-block elements The s-block elements exist in minerals: Albite(钠长石):Na[AISi,Og] Potash feldspar(钾长石):K[ASi,Og] Carnallite(光卤石):KCl.MgCl2·6H2O Alunite(明矾石):K(A1O)3(SO4)2·3H2O Spodumene(锂恽石):LiAl(SiO3)2
The s-block elements exist in minerals: Na AlSi 3 O8 K AlSi 3 O8 KClMgCl 2 6H2 O K(AlO) 3 (SO4 )2 3H2 O 3 2 Spodumene (锂辉石): LiAl(SiO ) Albite (钠长石): Potash feldspar (钾长石): Carnallite (光卤石): Alunite (明矾石): § 12.1.2 The existence and preparation of s-block elements

Beyl(绿柱石):Be3A12(SiO3)6 Magnesite(菱镁矿):MgCO; Gess0(石膏):CaSO4·2H2O Marble(大理石):CaCO Fluorite(萤石):CaF2 Celestite(天青石):SrSO4 Barite(重晶石):BaSO4
Beryl (绿柱石): Magnesite (菱镁矿): Fluorite (萤石): Celestite (天青石): Marble (大理石): 3 2 3 6 Be Al (SiO ) MgCO3 CaSO4 2H2 O CaCO3 CaF2 SrSO4 BaSO4 Gesso (石膏): Barite (重晶石):

Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. In addition,热还原法,金属置换法,热分解法etal (See page 465-466
Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. (See page 465-466 ) In addition, 热还原法, 金属置换法, 热分解法 et al
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 11 Basic of Coordination Chemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 10 Reduction - oxidization Reactions.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 9 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 8 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 7 Crystal Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 6 Molecular Structure and covalent bond theory.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 5 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 4 Chemical equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 2 Basic of thermodynamics.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 1 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 9 Molecular Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 8 Atomic Structure.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 7 Redox Reactions and the Base of Electrochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 16 The d-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 15 The p-block elements(Ⅲ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 14 Chapter 14 The p-block elements(Ⅱ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 13 The p-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 12 The s-Block Elements.pptx
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 11 Coordination Compound Structures.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 10 Solid Structure.pptx
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 13 The elements of boron family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 14 The elements of carbon family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 15 The elements of nitrogen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 16 The elements of oxygen family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 17 The halogens.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 18 Hydrogen and the rare gases.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 19 The elements of copper and zinc family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 21 The elements of chromium and Manganese family.pdf
- 北京化工大学:《无机化学》课程电子教案(课件讲稿,2015)Chapter 22 The elements of iron and platinium families.pdf
- 广东医科大学:《有机化学》课程教学资源(教学大纲)有机化学教学大纲(药学专业)Organic Chemistry.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第一章 绪论 Organic Chemistry(负责人:杨雪梅).pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第三章 烯烃 Alkenes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第二章 烷烃和环烷烃 Alkane and Cycloalkane.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第四章 炔烃和二烯烃 Alkynes and Dienes.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第五章 二烯烃 CnH2n-2.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第六章 立体化学基础.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第七章 芳香烃.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第九章 醛和酮 aldehyde and ketone.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十章 羧酸和取代羧酸.pdf
- 广东医科大学:《有机化学》课程电子教案(课件讲稿)第十二章 羧酸衍生物 Carboxylic acid derivatives.pdf
