《药理学》课程授课教案(讲义)Chapter 36 -41

Chapter 36 Synthetic antimicrobial drugs I.Sulfonamides Sulfonamide compounds were the first effective chemotherapeutic drugs to be employed systemically for the prevention and cure of bacterial infections in human beings.They originally came from azo dyes.Prontosil is the first sulfonamide used in clinical practice in 1930s Many sulfonamides continued being synthesized during the last century,some of which still have been used as yet 【Mechanism of antibacterial action】 Sulfonamides with antibacterial activity have a similar chemical structure to p-aminobenzoic acid (PABA).which is essential material for bacterial growth to synthesize folic acid under the catalysis of dihydropteroate synthase because the wall of bacteria is impermeable to folic acid In contrast,human beings can not synthesize folic acid and must obtain preformed folate to perform the transport of one-carbon unit in the synthesis of DNA.RNA.and proteins. Sulfonamides compete with PABA for the enzyme dihydropteroate synthase,thus preventing the synthesis of bacterial folic acid.As a result,they interfere with the last synthesis of purine and pyrimidine,as well as some amino acids(figure 36-1). P-Ar Fig36-1 Mechanism of action of sulfonamides and trimethoprim 【Antibacterial spectrum】
Chapter 36 Synthetic antimicrobial drugs I. Sulfonamides Sulfonamide compounds were the first effective chemotherapeutic drugs to be employed systemically for the prevention and cure of bacterial infections in human beings. They originally came from azo dyes. Prontosil is the first sulfonamide used in clinical practice in 1930s. Many sulfonamides continued being synthesized during the last century, some of which still have been used as yet. 【Mechanism of antibacterial action】 Sulfonamides with antibacterial activity have a similar chemical structure to p-aminobenzoic acid (PABA), which is essential material for bacterial growth to synthesize folic acid under the catalysis of dihydropteroate synthase because the wall of bacteria is impermeable to folic acid. In contrast, human beings can not synthesize folic acid and must obtain preformed folate to perform the transport of one-carbon unit in the synthesis of DNA, RNA, and proteins. Sulfonamides compete with PABA for the enzyme dihydropteroate synthase, thus preventing the synthesis of bacterial folic acid. As a result, they interfere with the last synthesis of purine and pyrimidine, as well as some amino acids (figure 36-1). Fig 36-1 Mechanism of action of sulfonamides and trimethoprim 【Antibacterial spectrum】

Sulfonamides are bacteriostatic and inhibit both gram-positive and gram-negative bacteria, nocardia,Chlamydia trachomatis,some protozoa,and some enteric bacteria,such as E.coli. klebsiella,salmonella,shigella,and enterobacter.However,rickettsiae are not inhibited by sulfonamides but are actually stimulated in their growth. 【Resistance】 Bacteriaal resistance to sulfonamides can arise from plasmid transfers random mutations. The resistance in general,irreversible and may be caused by any offollowing three possibilities:1) the affinity of dihydropteroate synthase for sulfonamides decreases,and thus they become less effective competitors than PABA for this enzyme:2)Permeability to sulfonamides may be decreased insome,3)bacteria increase the production of PABA 【Pharmacokinetics】 Sulfonamides can be divided into three groups:1)oral,absorbable,2)oral,non-absorbable,3) topical.The oral,absorbable sulfonamides can be further divided into short-medium-,and ong-acting groupson th their half-lives.Only medium-acting groups are stll widely used for some bacterial infections.Sulfonamides are distributed in tissues and body fluids.They are metabolized by acetylation or glucuronidation in the liver and then excreted via urine. 【Clinicaluse】 1.Oral absorbable group Sulfisoxazole (6h)and sulfamethoxazole (h)are used to treat urinary tract infections.Sulfadiazine(t 13h)achieves high concentrations in CSF. and can be used with penicillin G for Neiseria meningitis or with pyrimethamine for toxoplasmosis.Sulfadoxime (8d)is available in combination with pyrimethamine as second-line drug for malaria 2.Oral non-absorbable group Sulfasalazine [sAlfo'salazin]is widely used in ulcerative coliisentriisand other inflammatory boweldisase. 3.Topical group Sodium sulfacetylamide] onment is effective treament for bacterialoivis and as adjunctive therapy for trachoma Silver sulfadiazine is available for prevention of infection of burn wounds. 【Adverse reactions】 Sulfonamides have a variety of side reactions due partly to allergy and partly to toxicity of the active drugs and their metabolites.Up to5of patients exhibit untoward reactions
Sulfonamides are bacteriostatic and inhibit both gram-positive and gram-negative bacteria, nocardia, Chlamydia trachomatis, some protozoa, and some enteric bacteria, such as E.coli, klebsiella, salmonella, shigella, and enterobacter. However, rickettsiae are not inhibited by sulfonamides but are actually stimulated in their growth. 【Resistance】 Bacteriaal resistance to sulfonamides can arise from plasmid transfers or random mutations. The resistance in general, irreversible and may be caused by any of following three possibilities: 1) the affinity of dihydropteroate synthase for sulfonamides decreases, and thus they become less effective competitors than PABA for this enzyme; 2) Permeability to sulfonamides may be decreased in some resistant strains; 3) bacteria increase the production of PABA. 【Pharmacokinetics】 Sulfonamides can be divided into three groups: 1) oral, absorbable; 2) oral, non-absorbable; 3) topical. The oral, absorbable sulfonamides can be further divided into short- medium-, and long-acting groups on the their half-lives. Only medium-acting groups are still widely used for some bacterial infections. Sulfonamides are distributed in tissues and body fluids. They are metabolized by acetylation or glucuronidation in the liver and then excreted via urine. 【Clinical use】 1. Oral absorbable group Sulfisoxazole (t1/2 6h) and sulfamethoxazole (t1/2 11h) are used to treat urinary tract infections. Sulfadiazine (t1/2 13h) achieves high concentrations in CSF, and can be used with penicillin G for Neiseria meningitis or with pyrimethamine for toxoplasmosis. Sulfadoxine (t1/2 8d) is available in combination with pyrimethamine as second- line drug for malaria. 2. Oral non-absorbable group Sulfasalazine [slf’sælzin] is widely used in ulcerative colitis, enteritis, and other inflammatory bowel disease. 3. Topical group Sodium sulfacetylamide [sכdim slf’setilæmaid] ophthalmic solution or ointment is effective treatment for bacterial conjunctivitis and as adjunctive therapy for trachoma. Silver sulfadiazine is available for prevention of infection of burn wounds. 【Adverse reactions】 Sulfonamides have a variety of side reactions due partly to allergy and partly to toxicity of the active drugs and their metabolites. Up to 5 % of patients exhibit untoward reactions

including:1)allergic reactions and reactions with other sulfonamide derivatives. such as thiazides diuretics,sulfonaurea,diazoxide,furosemide,and bumetanide:2)crystalluria and hematuria,which may be decreased by adequate hydration and alkalinization of urine:3) hematopoietic disturbances.including hemolytic anemia in patients with G-6-PD deficiency. granulocytopenia,and thrombocytopenia I.Trimethoprim Trimethoprim is a potent inhibitor of bacterial dihydrofolate reductase,and prevents dihydrofolic acid to become tetrahydrofolic acid.It exhibits an antibacterial spectrum similar to the sulfonamides,but the antibacterial potency is 20 to50 time more than sulfonamide It is absorbed well after oral administration and has similar pharmacokinetic properties to sulfamethoxazole Therefore,it has been fommulated with sulfamethoxazole as co-rim to treat the patients with mild bacterial infections.Trimethoprim can produce the effects of folate deficiency,that may cause megaloblastic anemia leukopenia,and granulocytopenia IIL.Fluoquinolones Nalidixic acid is ancestor offluoqunoeswhich had been discarded in190sbecause of less effectivenes against bacteria and much more adverse reactions.However,since late1970 many fluoquinolones have been being developed in some pharmaceutic companies of the world. They quickly occupy the part of market of antimicrobial drugs. 【Mechanism of antibacterial action】 As well known that topoisomerase II is the enzyme that changes the configuration or topology of DNA by nicking pass-through,and re-sealing mechanism without changing DNA primary structure during the period of DNA replication.Topoisomerase IV plays action on separation of interlinked (catenated)daughter DNA molecules that are the product of DNA replication.DNA gyrase of bacteria is topoismerase II.Fluquinolones block bacterial DNA synthesis by inhibiting bacterial DNA gyrase and Topoisomerase IV.Thus,they interfere with the normal replication and transription of bacterial DNA at the conenraions of10gm and lead to cell death Eukaryotic cells do not contain DNA gyrase.But they contain a conceptually and mechanistically similar topoisomerase II that removes positive supercoils from
including: 1) allergic reactions and cross-allergic reactions with other sulfonamide derivatives, such as thiazides diuretics, sulfonaurea, diazoxide, furosemide, and bumetanide; 2) crystalluria and hematuria, which may be decreased by adequate hydration and alkalinization of urine; 3) hematopoietic disturbances, including hemolytic anemia in patients with G-6-PD deficiency, granulocytopenia, and thrombocytopenia. II. Trimethoprim Trimethoprim is a potent inhibitor of bacterial dihydrofolate reductase, and prevents dihydrofolic acid to become tetrahydrofolic acid. It exhibits an antibacterial spectrum similar to the sulfonamides, but the antibacterial potency is 20 to 50 time more than sulfonamide. It is absorbed well after oral administration and has similar pharmacokinetic properties to sulfamethoxazole. Therefore, it has been formulated with sulfamethoxazole as co-trimoxazole to treat the patients with mild bacterial infections. Trimethoprim can produce the effects of folate deficiency, that may cause megaloblastic anemia, leukopenia, and granulocytopenia. III. Fluoquinolones Nalidixic acid is ancestor of fluoquinolones, which had been discarded in 1960s because of less effectiveness against bacteria and much more adverse reactions. However, since late 1970s many fluoquinolones have been being developed in some pharmaceutic companies of the world. They quickly occupy the part of market of antimicrobial drugs. 【Mechanism of antibacterial action】 As well known that topoisomerase II is the enzyme that changes the configuration or topology of DNA by nicking, pass-through, and re-sealing mechanism without changing DNA primary structure during the period of DNA replication. Topoisomerase IV plays action on separation of interlinked (catenated) daughter DNA molecules that are the product of DNA replication. DNA gyrase of bacteria is topoisomerase II. Fluoquinolones block bacterial DNA synthesis by inhibiting bacterial DNA gyrase and Topoisomerase IV. Thus, they interfere with the normal replication and transcription of bacterial DNA at the concentrations of 0.1-10 g/ml and lead to cell death. Eukaryotic cells do not contain DNA gyrase. But they contain a conceptually and mechanistically similar topoisomerase II that removes positive supercoils from

eukaryotic DNA to prevent its tanging during replication.Fluoquinolones inhibit eukaryotic topoisomerase Ilony at the leves of 100-1000 Hg/ml). Fig 36-2 Model of the formation of negative DNA supercoils by DNA gyrase 【Antibacterial spectrum and use】 All fluoquinolones are bactericidal.Generally,they are effective against gram-negative bacteria such as enterobacteria,pseudomonas organisms,Haemophilus influenzae,Moraxella catarrhalis,Legionella.Chlamydia,and mycobacteria.They are effective in the treatment of gonorrhea but not syphilis Though active against some gram-positive bacteria,they should be avoided to treat the patients with pneumococcal or enterococcal infections.They have poor activity on anaerobes. 1.Ciprofloxacin Ciprofloxacin is the most potent of fluoquinolones and an good alternative to more toxic drugs,such as aminoglycosides It may has synergistical activity wth B-lactams against some bacterial infections and often effective in treatment of infections unresponsive to ampicillin,amoxicillin,and bacampicillin.Ciprofloxacin is often used in treament of rinary Itis highly in treatingacute enteric pathologens Ciprofloxacin also can be used for many infectious diseases due to gonorrhea.Enterobactor.E coli.Klebsiella pneumoniae.Haemophilus influerae.Legionella pneumophila.Pseudomonas aeruginosa.Proteus mirabilis.Serrana marcescens.and shigella But like other fluoquinoloesit has weak action on Streptococcus pneumoniae 2.Norfoxacin Norfloxacin has an antibacterial spectrum similar to that of ciprofloxacin and is used to treat the infectious diseases from gram-negative and gram-positive organisms. 3.Ofloxacin is primarily used in the treatment of prostatitis caused by E.co and of sxally transmitted diseases except syphilis.It may be used as alterative therapy in
eukaryotic DNA to prevent its tanging during replication. Fluoquinolones inhibit eukaryotic topoisomerase II only at the leves of 100-1000 g/ml). Fig 36-2 Model of the formation of negative DNA supercoils by DNA gyrase 【Antibacterial spectrum and clinical use】 All fluoquinolones are bactericidal. Generally, they are effective against gram-negative bacteria such as enterobacteria, pseudomonas organisms, Haemophilus influenzae, Moraxella catarrhalis, Legionella, Chlamydia, and mycobacteria. They are effective in the treatment of gonorrhea but not syphilis. Though active against some gram-positive bacteria, they should be avoided to treat the patients with pneumococcal or enterococcal infections. They have poor activity on anaerobes. 1. Ciprofloxacin Ciprofloxacin is the most potent of fluoquinolones and an good alternative to more toxic drugs,such as aminoglycosides. It may has synergistical activity wth -lactams against some bacterial infections and often effective in treatment of infections unresponsive to ampicillin, amoxicillin, and bacampicillin. Ciprofloxacin is often used in treatment of urinary infections. It is highly efficacious in treating acute diarrheal illnesses due to enteric pathologens. Ciprofloxacin also can be used for many infectious diseases due to gonorrhea, Enterobactor, E coli, Klebsiella pneumoniae, Haemophilus influenzae, Legionella pneumophila, Pseudomonas aeruginosa, Proteus mirabilis, Serratia marcescens, and shigella. But like other fluoquinolones, it has weak action on Streptococcus pneumoniae. 2. Norfloxacin Norfloxacin has an antibacterial spectrum similar to that of ciprofloxacin and is used to treat the infectious diseases from gram-negative and gram-positive organisms. 3. Ofloxacin Ofloxacin is primarily used in the treatment of prostatitis caused by E. coli and of sexually transmitted diseases except syphilis. It may be used as alternative therapy in

patients with gonorrhea.It has some benefit in the treatment of skin and lower respiratory tract infections. 4.Lomefloxacin and enoxacin Lomefloxacin and enoxacin are useful in the treatment of urethral tract infection and bronchitisdue to Haemoohilus influenzaeor Moraxella catarhalis. 5.Trovafloxacin(Trovafloxin)Trovafloxacin is anew fluoroquinolone,which has several properties that give it some clinical advantages.These propertics include a long half-life of about 10 hours,which permits on-a-day dosing.and a extended spetrum of antimicrobial activity including most anaerobes.However,trovafloxacin has aserious toxicity of liver injury. and use of the drug is restricted to infections that are life-threatening (for example,pneumonia, intra-abdominal infections,gynecologic and pelvic infections).The therapy with trovafloxacin should not for longer than 14 days.anf should only be used in hospital and long-term nursing care facilities 【Resistance】 When the were first introduced,there was optimism that resistance would not develop.Although no plasmid-mediated resistance has been reported,esistance of MRSA,pseudomonas coagulase-negative staphylococci and enterococci has unfortunately emerged due to chromosomal mutations.Cross-resistance exists among the quinolones.The mechanisms responsible for this resistance are:1)Modifications in the bacterial DNA gyrase. especially in amino acids at the N-terminus of the A subunit,have been associated with decreased affinity for fluoroquinolones.2)Accumulation of fluoroquinolones in bacteria decreases because of a decreased number of porin proteins in the outer membrane and disorder of an energy-dependent efflux system. 【Pharmacokinetics】 After oral administration,fluoroquinoloes are well absorbed (bioavailability of8%)and distributed widely in body fluids and tissues.Serun elinination half-lives rangs from3 hours to 18 hours (Table 36-1).Oral absorption is impaired by divalent cations.Most of fluoroquinolones are eliminated by kidneys
patients with gonorrhea. It has some benefit in the treatment of skin and lower respiratory tract infections. 4. Lomefloxacin and enoxacin Lomefloxacin and enoxacin are useful in the treatment of urethral tract infection and bronchitis due to Haemoohilus influenzae or Moraxella catarrhalis. 5. Trovafloxacin (Trovafloxin) Trovafloxacin is anew fluoroquinolone, which has several properties that give it some clinical advantages. These properties include a long half-life of about 10 hours, which permits once-a-day dosing, and a extended spetrum of antimicrobial activity including most anaerobes. However, trovafloxacin has a serious toxicity of liver injury, and use of the drug is restricted to infections that are life-threatening (for example, pneumonia, intra-abdominal infections, gynecologic and pelvic infections). The therapy with trovafloxacin should not continue for longer than 14 days, anf should only be used in hospital and long-term nursing care facilities. 【Resistance】 When the fluoroquinolones were first introduced, there was optimism that resistance would not develop. Although no plasmid-mediated resistance has been reported, esistance of MRSA, pseudomonas, coagulase-negative staphylococci and enterococci has unfortunately emerged due to chromosomal mutations. Cross-resistance exists among the quinolones. The mechanisms responsible for this resistance are: 1) Modifications in the bacterial DNA gyrase, especially in amino acids at the N-terminus of the A subunit, have been associated with decreased affinity for fluoroquinolones. 2) Accumulation of fluoroquinolones in bacteria decreases because of a decreased number of porin proteins in the outer membrane and disorder of an energy-dependent efflux system. 【Pharmacokinetics】 After oral administration, fluoroquinolones are well absorbed (bioavailability of 80-95%) and distributed widely in body fluids and tissues. Serun elinination half-lives rangs from 3 hours to 18 hours (Table 36-1). Oral absorption is impaired by divalent cations. Most of fluoroquinolones are eliminated by kidneys
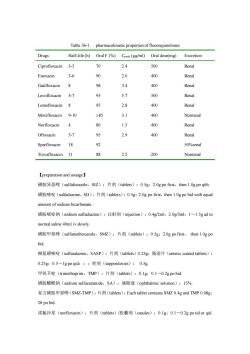
Table 36-1.pharmacokinetic properties of fluoroquinolones Drugs Half-life(h)Oral F(%)Cpeak(ug/ml)Oral dose(mg)Excretion Ciprofloxacin 3-5 70 2.4 500 Renal Enoxacin 3-6 90 2.0 400 Renal Gatifloxacin 8 98 3.4 400 Renal Levofloxacin 5-7 95 5.7 500 Renal Lomefloxacin 8 95 2.8 400 Renal Moxifloxacin 9-10 >85 3.1 400 Nonrenal Norfloxacin 80 1.5 400 Renal Ofloxacin 5-7 95 2.9 400 Renal Sparfloxacin 18 92 50%renal Trovafloxacin 11 88 2.2 200 Nonrenal 【preparation and usuage】 磺胺异恶唑(sulfafurazole,SZ):片剂(tablets):0.5g:2.0 gpofirst,then 1.0gpoq6h 磺胺密啶(sulfadiazine,SD):片剂(tablets):0.5g:2.0 g po first,then 1.0 gpo bid with equal amount of sodium bicarbonate. 磺胺密啶钠(sodium sulfadiazine):注射剂(injection):0.4g/2ml,2.0g/5ml:1~1.5 g ad to normal saline 40ml ivslowly. 磺胺甲恶唑(sulfamethoxazole,SM亿):片剂(tablets):0.5g:20 g po first,then1.0gpo bid. 柳氨磺嘧啶(sulfasalazine,SASP):片剂(tablets)0.2Sg:肠溶片(enteric coaed tabes): 0.25g:0.5~lg poqid:::栓剂(suppositories):0.5g 甲氧苄啶(trimethoprim,TMP):片剂(tablets):0.1g:0.1~0.2 g po bid. 磺胺醋酰钠(sodium sufacetamide,SA):滴眼液(ophthamicsotion:15%, 复方磺胺甲恶唑(SM亿-TMP):片剂(tablets):Each tablet contains SMZ0.4 gand TMP0.08g: 2#po bid. 诺氟沙星(norfloxacin):片剂(tablets)/胶囊剂(casules):0.1g:0.1~0.2 g potid or qid
Table 36-1. pharmacokinetic properties of fluoroquinolones Drugs Half-life (h) Oral F (%) Cpeak (g/ml) Oral dose(mg) Excretion Ciprofloxacin Enoxacin Gatifloxacin Levofloxacin Lomefloxacin Moxifloxacin Norfloxacin Ofloxacin Sparfloxacin Trovafloxacin 3-5 3-6 8 5-7 8 9-10 4 5-7 18 11 70 90 98 95 95 85 80 95 92 88 2.4 2.0 3.4 5.7 2.8 3.1 1.5 2.9 2.2 500 400 400 500 400 400 400 400 200 Renal Renal Renal Renal Renal Nonrenal Renal Renal 50%renal Nonrenal 【preparation and usuage】 磺胺异恶唑(sulfafurazole,SIZ):片剂(tablets):0.5g;2.0g po first,then 1.0g po q6h. 磺胺嘧啶(sulfadiazine,SD):片剂(tablets):0.5g;2.0g po first,then 1.0g po bid with equal amount of sodium bicarbonate. 磺胺嘧啶钠(sodium sulfadiazine):注射剂(injection):0.4g/2ml,2.0g/5ml;1~1.5g ad to normal saline 40ml iv slowly. 磺胺甲恶唑(sulfamethoxazole,SMZ):片剂(tablets):0.5g;2.0g po first, then 1.0g po bid. 柳氮磺嘧啶(sulfasalazine,SASP):片剂(tablets)0.25g;肠溶片(enteric coated tablets): 0.25g;0.5~1g po qid;:;栓剂(suppositories): 0.5g. 甲氧苄啶(trimethoprim,TMP):片剂(tablets):0.1g;0.1~0.2g po bid. 磺胺醋酰钠(sodium sulfacetamide,SA):滴眼液(ophthalmic solution):15%. 复方磺胺甲恶唑(SMZ-TMP):片剂(tablets):Each tablet contains SMZ 0.4g and TMP 0.08g; 2# po bid. 诺氟沙星(norfloxacin):片剂(tablets)/胶囊剂(casules):0.1g;0.1~0.2g po tid or qid

氧氟沙星(ofloxacin):片剂(tablets):0.lg:0.1~0.3 g po bid.注射剂(injection):0.4g10ml: 0.4g iv gtt q12h after diluted. 培氟沙星(perfloxacin):片剂((tablets):0.2g:0.4 gpo bid.注射剂((injection):0.4g/5ml: 0.4g iv gtt qhafter diluted with5%GS 250ml. 依诺沙星(enoxacin):片剂(tablets)/胶囊剂(casules):0.1g:0.2~0.3 gpobid. 环丙沙星(ciprofloxacin):片剂(tablets)/胶囊剂(casules):0.1g:0.25g,0.5g,0.75g: 0.25~0.5g po bid (injection):0.1g/100ml,0.2g/200ml:0.1~0.2giv gut after diluted with normal salineor%GS 左氧氟沙星(1 evofloxacin):片剂(tablets):0.1g:0.1~0.2 g po bid.注射剂(injection): .g/100ml,0.3g/100ml:0.2g ivgtt q2hafter diluted. 洛美沙星(lomefloxacin):簿膜衣片((flm tablets):0.4g:0.4 g poqd.注射剂(injection): .g/100ml.g/50ml:0.givgtr4givgqd after diluted. 司氟沙星(sparfloxacin):胶囊剂(capsules):0.lg:0.1~0.3gqd 加替沙星(gatifloxacin):片剂(tablets):0.lg,0.2g,0.4g:0.2~0.4 g po qd.注射剂(injection): 0.Ig/100ml,0.2g/100ml:.~.4giv gtt qd after diluted. (Q-X-Zhou)
氧氟沙星(ofloxacin):片剂(tablets):0.1g;0.1~0.3g po bid. 注射剂(injection):0.4g/10ml; 0.4g iv gtt q12h after diluted. 培氟沙星(perfloxacin):片剂(tablets):0.2g;0.4g po bid. 注射剂(injection):0.4g/5ml; 0.4g iv gtt q12h after diluted with 5% GS 250ml. 依诺沙星(enoxacin):片剂(tablets)/胶囊剂(casules):0.1g;0.2~0.3g po bid. 环丙沙星(ciprofloxacin):片剂(tablets)/胶囊剂(casules):0.1g;0.25g,0.5g,0.75g; 0.25~0.5g po bid. 注射剂(injection):0.1g/100ml,0.2g/200ml;0.1~0.2g iv gtt after diluted with normal saline or 5% GS. 左氧氟沙星(levofloxacin):片剂(tablets):0.1g;0.1~0.2g po bid. 注射剂(injection): 0.2g/100ml,0.3g/100ml;0.2g iv gtt q12h after diluted. 洛美沙星(lomefloxacin):薄膜衣片(film tablets):0.4g;0.4g po qd. 注射剂(injection): 0.2g/100ml,0.4g/250ml;0.2g iv gtt q12h or 0.4g iv gtt qd after diluted. 司氟沙星(sparfloxacin):胶囊剂(capsules):0.1g;0.1~0.3g qd. 加替沙星(gatifloxacin):片剂(tablets):0.1g,0.2g,0.4g;0.2~0.4g po qd. 注射剂(injection): 0.1g/100ml,0.2g/100ml;0.2~0.4g iv gtt qd after diluted. (Q-X-Zhou)

Chapter 37 Antifungal drugs Human fungal have inereased dramaticaly in recent years,due to surgery,cancer treatment,and critical care with the use of broad-spectrum antimicrobials as well as the HIV epidemic. Fungal infections traditionally are divided into two distinct classes:systemic and superficial. Conseqently.the mjor antifungal agents describd in this chapter fall into systemic drugs and topical drugs. Amphotericin B Amphotericin B was discovered in 956 by Gold and co-workers who were studying a strain of Srreplomyces nod an aerobic actinomycete.obtained from the Orinoco River Valley of Venezuela Asa polyen macrolide antibiotic,amphotericin B is unstable at room temperature. 【Antifungal Activity and Mechanism】
Chapter 37 Antifungal drugs Human fungal infections have increased dramatically in recent years, due to surgery, cancer treatment, and critical care with the use of broad-spectrum antimicrobials as well as the HIV epidemic. Fungal infections traditionally are divided into two distinct classes:systemic and superficial. Conseqently, the major antifungal agents described in this chapter fall into systemic drugs and topical drugs. Amphotericin B Amphotericin B was discovered in 1956 by Gold and co-workers who were studying a strain of Streptomyces nodosus, an aerobic actinomycete, obtained from the Orinoco River Valley of Venezuela. As a polyen macrolide antibiotic, amphotericin B is unstable at room temperature. 【Antifungal Activity and Mechanism】

Amphotericin B has the broadest spectrum of antifungi activity,including Candida albicans and Cryptococcus neofimans that cause endemic mycoses(Blastomyces dermatitidis.Histoplasma capsulatm.Coccidioides immitis).and the pathogenic molds,such as Aspergillus fumigats and mcor.Some fungal organisms such as Candida lusitaniae and Pseudallescheria boydii display intrinsic amphotericin B resistance. Antifungal activity of amphotericin B depends at least partly on its binding to a sterol moiety. primarily ergosterol existing in the membrane of sensitive fungi.By virtue of the interaction with the sterols of cell membranes,amphotericin B makes membrane form pores or channels which allowing leakage ofa variety of small molecules At last,the fungi are dead. 【Clinical Use】 Amphotericin B is the drug of choice for treating systemic mycoses in spite of its toxic potential.It is sometimes used in combination with flucytosine so that lower levels of amphotericin B are possible in order to relieve the toxicity.amphotericin B is given by intravenous infusion. 【Adverse Effects】 Amphotericin B has a low therapeutic index.A total daily dose should not exceed 1.5 mg/kg Small test dose is usually administered to assess the degree of a patient's negative response After infusion,it can cause high fever,chills,muscle spasms,vomiting headache,and hypotension,which could be ameliorated by slowing infusion rate and decreasing daily dose,or premedication with glucocorticosteroids or antipyretics.Renal damage is the most significant after it is used time.Renal impairment curs in nearly all patients reated with clinically significant doses of amphotericin Aminoglycosides,cyclosporine,and pentamidine exacerbate the renal damage. Azoles Azoles include imdazoles and triazoles.The imidazoles consist of ketoconazole, miconazole,and clotrimazole.These three drugs are used mainly in topical therapy.The triazoles include itraconazole and fluconazole both of which are in common use for systemic treatment of fungal disease
Amphotericin B has the broadest spectrum of antifungi activity, including Candida albicans and Cryptococcus neoftmans that cause endemic mycoses (Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis), and the pathogenic molds, such as Aspergillus fumigatus and mucor. Some fungal organisms such as Candida lusitaniae and Pseudallescheria boydii display intrinsic amphotericin B resistance. Antifungal activity of amphotericin B depends at least partly on its binding to a sterol moiety, primarily ergosterol existing in the membrane of sensitive fungi. By virtue of the interaction with the sterols of cell membranes, amphotericin B makes membrane form pores or channels, which allowing leakage of a variety of small molecules. At last, the fungi are dead. 【Clinical Use】 Amphotericin B is the drug of choice for treating systemic mycoses in spite of its toxic potential. It is sometimes used in combination with flucytosine so that lower levels of amphotericin B are possible in order to relieve the toxicity. amphotericin B is given by intravenous infusion. 【Adverse Effects】 Amphotericin B has a low therapeutic index. A total daily dose should not exceed 1.5 mg/kg. Small test dose is usually administered to assess the degree of a patient’s negative response. After infusion, it can cause high fever, chills, muscle spasms, vomiting, headache, and hypotension, which could be ameliorated by slowing infusion rate and decreasing daily dose, or premedication with glucocorticosteroids or antipyretics. Renal damage is the most significant toxic reaction after it is used for long time. Renal impairment occurs in nearly all patients treated with clinically significant doses of amphotericin. Aminoglycosides, cyclosporine, and pentamidine exacerbate the renal damage. Azoles Azoles include imdazoles and triazoles. The imidazoles consist of ketoconazole, miconazole, and clotrimazole. These three drugs are used mainly in topical therapy. The triazoles include itraconazole and fluconazole, both of which are in common use for systemic treatment of fungal disease

Ketoconazole Ketoconazole is used in the treatment of many kinds of mucocutaneous candidiasis.Topical and shampoo forms of ketoconazole are also available and useful in the treatment of seborheic dermatitis and pityriasis versicolor.It is easy to be absorbed at a low gastric PH.Because of liver toxicity,it is limited for systemic treatment.Ketoconazole may cause gastrointestinal upset,abnormity of liver function,and synthetic disorders of androgen and adrenal steroids. etraconazole is available in an oral formulation and its absorption is increased by food and by low gastric PH.Itraconazole isa drag of choice in the treatment of dermatopyoses and onchomcos and is the only agent with significant activity against aspergillus secies. isrelatively lowtoxic.The is upset. Fluconazole The drug is available in oral and intravenous formulations.Renal excretion accounts for over%of elimination,and the elimination half-life is25to30 hours It is used for treating candidiasis.cryplococcosis and AIDS patients with cryptococcal meningitis.The and dizziness may be seen. Clotrimazole and miconazole Clotrimazole and miconazole are often used for vulvovaginal candidiasis.In cream form,both agents are useful for dermatophytic infections.Absorption is neglible,and adverse effects are rare. Griseofulvin Its only use is in the systemic treatment of dermatophytosis.It is available in an oral formulation and absorption is improved when it is given with fatty foods.Its action is to prevent new infection of skin structures It must be administered for at least -6weeks for skin and hair infections to allow the replacement of infected keratin by the resistant structures Adverse effects include allergic syndrome,hepatitis,and drug interactions with warfarin. Griseofulvin has been largely replaced by newer antifungal medicationsschastacand terbinafine. Terbinafine It can be taken orally and used in treatment of dermatophytoses,especially onychomycosis.Like azole drugs,it interferes with ergosterol biosynthesis but rather than interacting with the P450 system.Adverse effects are rare,consisting primarily of gastrointestinal upset and headache
Ketoconazole Ketoconazole is used in the treatment of many kinds of mucocutaneous candidiasis. Topical and shampoo forms of ketoconazole are also available and useful in the treatment of seborrheic dermatitis and pityriasis versicolor. It is easy to be absorbed at a low gastric PH. Because of liver toxicity, it is limited for systemic treatment. Ketoconazole may cause gastrointestinal upset, abnormity of liver function, and synthetic disorders of androgen and adrenal steroids. Itraconazole Itraconazole is available in an oral formulation and its absorption is increased by food and by low gastric PH. Itraconazole is a drag of choice in the treatment of dermatophytoses and onychomycosis and is the only agent with significant activity against aspergillus species. Itraconazole is relatively low toxic. The most common adverse reaction is gastrointestinal upset. Fluconazole The drug is available in oral and intravenous formulations. Renal excretion accounts for over 90% of elimination, and the elimination half-life is 25 to 30 hours. It is used for treating candidiasis, cryptococcosis and AIDS patients with cryptococcal meningitis. The adverse effects of fluconazole is relatively minor in same kinds, alimentary canal upset, headache and dizziness may be seen. Clotrimazole and miconazole Clotrimazole and miconazole are often used for vulvovaginal candidiasis. In cream form, both agents are useful for dermatophytic infections. Absorption is negligible, and adverse effects are rare. Griseofulvin Its only use is in the systemic treatment of dermatophytosis. It is available in an oral formulation and absorption is improved when it is given with fatty foods. Its action is to prevent new infection of skin structures. It must be administered for at least 2-6 weeks for skin and hair infections to allow the replacement of infected keratin by the resistant structures. Adverse effects include allergic syndrome, hepatitis, and drug interactions with warfarin. Griseofulvin has been largely replaced by newer antifungal medications such as itraconazole and terbinafine. Terbinafine It can be taken orally and used in treatment of dermatophytoses, especially onychomycosis. Like azole drugs, it interferes with ergosterol biosynthesis, but rather than interacting with the P450 system. Adverse effects are rare, consisting primarily of gastrointestinal upset and headache
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 重庆医科大学:《药理学》课程教学大纲(72学时).doc
- 《药物分析》课程教学资源(PPT课件)药物分析实验讲稿.ppt
- 《药物分析》课程教学资源(PPT课件)第16章 药品质量控制中现代分析方法的进展.ppt
- 《药物分析》课程教学资源(PPT课件)第15章 药品质量标准的制定.ppt
- 《药物分析》课程教学资源(PPT课件)第14章 中药制剂分析.ppt
- 《药物分析》课程教学资源(PPT课件)第13章 生物制品分析概论.ppt
- 《药物分析》课程教学资源(PPT课件)第12章 制剂分析.ppt
- 《药物分析》课程教学资源(PPT课件)第11章 抗生素类药物分析 The Analysis of Aantibiotic drugs.ppt
- 《药物分析》课程教学资源(PPT课件)第10章 甾体激素类药物分析 The analysis of Steroid Hormon Drugs.ppt
- 《药物分析》课程教学资源(PPT课件)第9章 维生素类药物分析 The Analysis of Vitamins.ppt
- 《药物分析》课程教学资源(PPT课件)第8章 杂环类药物分析 The Analysis of Heterocyclic drugs.ppt
- 《药物分析》课程教学资源(PPT课件)第6章 芳酸及其酯类药物的分析.ppt
- 《药物分析》课程教学资源(PPT课件)第5章 巴比妥类药物的分析 Analysis of Barbitals Drugs.ppt
- 《药物分析》课程教学资源(PPT课件)第4章 药物定量分析与分析方法验证.ppt
- 《药物分析》课程教学资源(PPT课件)第3章 药物的杂质检查.ppt
- 《药物分析》课程教学资源(PPT课件)第1章 药典概况.ppt
- 《药物分析》课程教学资源(PPT课件)绪论 Pharmaceutical Analysis(重庆医科大学:范琦).ppt
- 《药物分析》课程试卷习题(含答案)药物分析习题二.doc
- 《药物分析》课程试卷习题(含答案)药物分析习题三.doc
- 《药物分析》课程试卷习题(含答案)药物分析习题四.doc
- 《药理学》课程教学资源(作业习题)习题集一(习题).doc
- 《药理学》课程教学资源(作业习题)习题集三(英文,习题).doc
- 《药理学》课程教学资源(作业习题)习题集二(习题).doc
- 《药理学》课程教学资源(作业习题)习题集一(参考答案).doc
- 《药理学》课程教学资源(作业习题)习题集二(参考答案).doc
- 《药理学》课程教学资源(作业习题)习题集三(英文,参考答案).doc
- 《药理学》课程授课教案(讲义)抗结核病、抗疟药.doc
- 《药理学》课程授课教案(讲义)抗阿米巴药、抗恶性肿瘤药.doc
- 《药理学》课程授课教案(讲义)合成抗菌药、抗真菌药.doc
- 《药理学》课程授课教案(讲义)作用于血液系统药物.doc
- 《药理学》课程授课教案(讲义)抗高血压药物.doc
- 《药理学》课程授课教案(讲义)调血脂药物.doc
- 《药理学》课程授课教案(讲义)抗心绞痛药物.doc
- 《药理学》课程授课教案(讲义)抗心律失常药物.doc
- 《药理学》课程授课教案(讲义)利尿药物.doc
- 《药理学》课程授课教案(讲义)强心药物.doc
- 《药理学》课程授课教案(讲义)肾上腺皮质激素药物.doc
- 《药理学》课程授课教案(讲义)镇痛解热镇痛.doc
- 《药理学》课程授课教案(讲义)抗生素.doc
- 《药理学》课程授课教案(讲义)抗慢性心功能不全.doc
