《分析化学》课程教学资源(课件讲稿,英文)Chapter 4

归东理工大军 Analytical Chemistry To master the requirements of the titration error; To know about the classical applications and calculation of all kinds of titrations. 2022/10/26 2
Analytical Chemistry ØTo master the requirements of the titration error; Ø To know about the classical applications and calculation of all kinds of titrations. 2022/10/26 2

G 归东龙子大图 Analytical Chemistry $4.1 Acid-base equilibria 1.Acid-base theory The acidity or basicity of a solution is frequently an important factor in chemical reactions.Fundamental acid-base equilibria are important in understanding acid-base titration and the effect of acids on chemical species and reactions. According to Bronsted theory: An acid is a substance that can give up protons; A base is any compound or ion that can accept protons. 2022/10/26
Analytical Chemistry §4.1 Acid-base equilibria 1. Acid-base theory The acidity or basicity of a solution is frequently an important factor in chemical reactions. Fundamental acid-base equilibria are important in understanding acid-base titration and the effect of acids on chemical species and reactions. According to Bronsted theory: ØAn acid is a substance that can give up protons; ØA base is any compound or ion that can accept protons. 2022/10/26 3

归东理工大军 Analytical Chemistry A strong acid or base is completely dissociated in aqueous solution; A weak acid is one that is only partially dissociated in water,all weak acids HA react with water by donating a proton to h2O: HA+H,OU H.O+A The acid dissociation constant is K,H][A】 [HA] 2022/10/26
Analytical Chemistry ØA strong acid or base is completely dissociated in aqueous solution; ØA weak acid is one that is only partially dissociated in water, all weak acids HA react with water by donating a proton to H2O: The acid dissociation constant is 2022/10/26 4
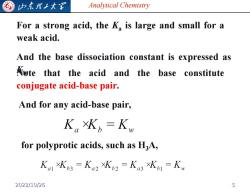
G 归东理子大溪 Analytical Chemistry For a strong acid,the k is large and small for a weak acid. And the base dissociation constant is expressed as Aute that the acid and the base constitute conjugate acid-base pair. And for any acid-base pair, K。Kb=K for polyprotic acids,such as H3A, Kal xKb3 =Ka2 xKb2 =Ka3 XKo1=Kw 2022/10/26 5
Analytical Chemistry For a strong acid, the Ka is large and small for a weak acid. And the base dissociation constant is expressed as Kb Note . that the acid and the base constitute conjugate acid-base pair. And for any acid-base pair, for polyprotic acids, such as H3A, 2022/10/26 5
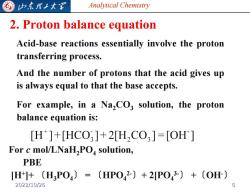
归东龙工大军 Analytical Chemistry 2.Proton balance equation Acid-base reactions essentially involve the proton transferring process. And the number of protons that the acid gives up is always equal to that the base accepts. For example,in a Na,CO3 solution,the proton balance equation is: [H]+[HCO3]+2[HCO3]=[OH] For c mol/LNaH2PO solution, PBE [H]+〔H3P04)=(HP042-)+2P043-)+〔OH) 2022/10/26
Analytical Chemistry 2. Proton balance equation Acid-base reactions essentially involve the proton transferring process. And the number of protons that the acid gives up is always equal to that the base accepts. For example, in a Na2CO3 solution, the proton balance equation is: For c mol/LNaH2PO4 solution, PBE [H+ ]+ 〔H3PO4〕 = 〔HPO4 2-〕+ 2[PO4 3-〕 +〔OH-〕 2022/10/26 6
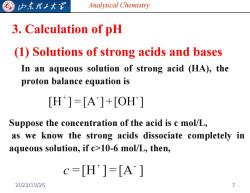
G 归东我子大军 Analytical Chemistry 3.Calculation of pH (1)Solutions of strong acids and bases In an aqueous solution of strong acid (HA),the proton balance equation is [H]=[A]+[OH] Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution,if c>10-6 mol/L,then, c=[H]=[A] 2022/10/26
Analytical Chemistry 3. Calculation of pH (1) Solutions of strong acids and bases In an aqueous solution of strong acid (HA), the proton balance equation is Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution, if c>10-6 mol/L, then, 2022/10/26 7

归东龙工大军 Analytical Chemistry if c<10-6 mol/L, The autoprotolysis of water should not be neglected, [H]=c+ H] [H1=c+e+4K 2 For strong bases,similar equations can be derived for calculation of [OH-]. 2022/10/26
Analytical Chemistry if c<10-6 mol/L, The autoprotolysis of water should not be neglected, For strong bases, similar equations can be derived for calculation of [OH- ]. 2022/10/26 8

G 归东理子大图 Analytical Chemistry (2)Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant,K.Similarly,the pH of a weak base can be calculated from its Kp value. For a strong bases,similar equations can be derived for calculation of [OH-]. For a weak acid,HA,we can write its proton balance equation: H]=[A]+[OH] 2022/10/26
Analytical Chemistry (2) Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant, Ka . Similarly, the pH of a weak base can be calculated from its Kb value. For a strong bases, similar equations can be derived for calculation of [OH- ]. For a weak acid, HA, we can write its proton balance equation: 2022/10/26 9
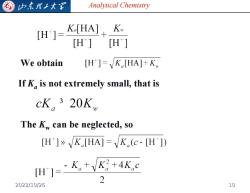
归东理工大 Analytical Chemistry H']= Ko[HA]K [H] [H] We obtain [H']=K[HA]+K If K,is not extremely small,that is cK。320K The K can be neglected,so [H']K[HA]=K.(e-[H']) H=K+K+4K,c 2 2022/10/26 10
Analytical Chemistry We obtain If Ka is not extremely small, that is The Kw can be neglected, so 2022/10/26 10
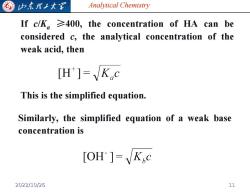
G 归东龙子大图 Analytical Chemistry If c/K,≥400,the concentration of HA can be considered c,the analytical concentration of the weak acid,then [H']=Kc This is the simplified equation. Similarly,the simplified equation of a weak base concentration is [OH ]=K,c 2022/10/26 11
Analytical Chemistry If c/Ka ≥400, the concentration of HA can be considered c, the analytical concentration of the weak acid, then This is the simplified equation. Similarly, the simplified equation of a weak base concentration is 2022/10/26 11
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 5 Complexometric Titration.ppt
- 《分析化学》课程教学资源(课件讲稿,英文)Chapter 6 Oxidation-reduction titration.pdf
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 7 precipitation titration.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 8 visible spectrophotometry.ppt
- 《分析化学》课程课后教学资源(实验预习指导)天平.doc
- 《分析化学》课程课后教学资源(实验预习指导)滴定分析练习.doc
- 《分析化学》课程课后教学资源(实验预习指导)有机酸摩尔质量.doc
- 《分析化学》课程课后教学资源(实验预习指导)水硬度.doc
- 《分析化学》课程课后教学资源(实验预习指导)胃舒平中铝镁含量的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)COD的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)分光光度法测铁.doc
- 《分析化学》课程课后思考题(含答案)第一章.doc
- 《分析化学》课程课后思考题(含答案)第二章.doc
- 《分析化学》课程课后思考题(含答案)第三章.doc
- 《分析化学》课程课后思考题(含答案)第五章.doc
- 《分析化学》课程课后思考题(含答案)第六章.doc
- 《分析化学》课程课后思考题(含答案)第七章.doc
- 《分析化学》课程课后思考题(含答案)第八章.doc
- 《分析化学》课程课后思考题(含答案)第十章.doc
- 《分析化学》课程课后习题(含答案)第三章.doc
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 3 summarization of Titrimetric analysis.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 2 Errors and data treatment in quantitative analysis.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 1 The classification of analytical chemistry.ppt
- 《分析化学》课程教学资源(试题,含答案)分析化学试题10.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题9.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题8.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题7.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题6.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题5.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题4.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题3.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题2.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题1.doc
- 《分析化学》课程教学资源(知识拓展)自来水中余氯含量的测定.doc
- 《分析化学》课程教学资源(文献资料)一种快速简便测定奶粉中蛋白质的方法.pdf
- 《分析化学》课程教学资源(知识拓展)禁止化学武器组织获诺贝尔和平奖.doc
- 《分析化学》课程教学资源(知识拓展)碘盐中碘含量的测定.doc
- 《分析化学》课程教学资源(知识拓展)PM2.5的测定方法.doc
- 《分析化学》课程教学课件(PPT讲稿)沉淀滴定和重量分析.ppt
- 《分析化学》课程教学课件(PPT讲稿)氧化还原滴定法(Oxidation-Reduction Titration).ppt
