《分析化学》课程教学课件(PPT讲稿,英文)Chapter 2 Errors and data treatment in quantitative analysis

G 归东理子大 Analytical Chemistry Chapter 2 Errors and data treatment in quantitative analysis
Analytical Chemistry 2025/4/3 1 Chapter 2 Errors and data treatment in quantitative analysis

归东理子大军 Analytical Chemistry Learning Objective To master the types,characteristics and expression methods of errors; ◆To master the definitions and applications of the precision and the accuracy,and also to understand the relationship between the precision and the accuracy;
Analytical Chemistry 2025/4/3 2 Learning Objective ◆To master the types, characteristics and expression methods of errors; ◆ To master the definitions and applications of the precision and the accuracy, and also to understand the relationship between the precision and the accuracy;

归东觅子大图 Analytical Chemistry To know about the distribution rules of random error,and master the definitions, functions and calculating methods of confidence levels and confidence intervals; To know about the evaluation methods of the quantitative data,realize the importance of improving the accuracy of analytical results;
Analytical Chemistry 2025/4/3 3 ◆ To know about the distribution rules of random error, and master the definitions, functions and calculating methods of confidence levels and confidence intervals; ◆ To know about the evaluation methods of the quantitative data, realize the importance of improving the accuracy of analytical results;

归东理子大军 Analytical Chemistry To master the ways and methods for improving the accuracy of analytical results; To know about the concept of significant figures, and master the rules of the computing and arithmetic rounding off
Analytical Chemistry 2025/4/3 4 ◆ To master the ways and methods for improving the accuracy of analytical results; ◆ To know about the concept of significant figures, and master the rules of the computing and arithmetic rounding off
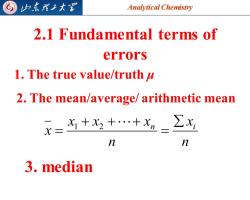
G 归东觅子大图 Analytical Chemistry 2.1 Fundamental terms of errors 1.The true value/truth u 2.The mean/average/arithmetic mean X= 为+x++x2=∑出 n n 3,median
Analytical Chemistry 2025/4/3 5 2.1 Fundamental terms of errors 1. The true value/truth μ 2. The mean/average/ arithmetic mean n x n x x x x n i = + + + = 1 2 3. median
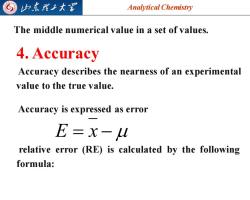
山东理子大军 Analytical Chemistry The middle numerical value in a set of values. 4.Accuracy Accuracy describes the nearness of an experimental value to the true value. Accuracy is expressed as error E=x- relative error (RE)is calculated by the following formula:
Analytical Chemistry 2025/4/3 6 4. Accuracy E = x − Accuracy is expressed as error relative error (RE) is calculated by the following formula: The middle numerical value in a set of values. Accuracy describes the nearness of an experimental value to the true value
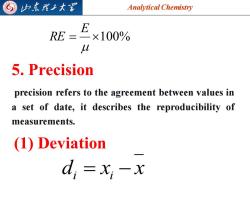
G 归东理子大图 Analytical Chemistry E RE=×100% U 5,Precision precision refers to the agreement between values in a set of date,it describes the reproducibility of measurements. (1)Deviation d;=xi-x
Analytical Chemistry 2025/4/3 7 = 100% E RE 5. Precision precision refers to the agreement between values in a set of date, it describes the reproducibility of measurements. (1) Deviation d x x i = i −

归东理王大军 Analytical Chemistry (2)Relative deviation RD=4×100% X (③)average deviation Σx-刘 d= n
Analytical Chemistry 2025/4/3 8 (2) Relative deviation = 100% x d RD i (3) average deviation n x x d n i i = − = 1

归东理子大军 Analytical Chemistry (4)standard deviation n-1 6.The relationship between accuracy and precision Error and deviation,the respective measures of accuracy and precision,are not always numerically related to one another
Analytical Chemistry 2025/4/3 9 (4) standard deviation ( ) 1 1 2 − − = = n x x s n i i 6. The relationship between accuracy and precision Error and deviation, the respective measures of accuracy and precision, are not always numerically related to one another

山东理工大军 Analytical Chemistry That is,the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy
Analytical Chemistry 2025/4/3 10 That is, the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 3 summarization of Titrimetric analysis.ppt
- 《分析化学》课程教学资源(课件讲稿,英文)Chapter 4.pdf
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 5 Complexometric Titration.ppt
- 《分析化学》课程教学资源(课件讲稿,英文)Chapter 6 Oxidation-reduction titration.pdf
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 7 precipitation titration.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 8 visible spectrophotometry.ppt
- 《分析化学》课程课后教学资源(实验预习指导)天平.doc
- 《分析化学》课程课后教学资源(实验预习指导)滴定分析练习.doc
- 《分析化学》课程课后教学资源(实验预习指导)有机酸摩尔质量.doc
- 《分析化学》课程课后教学资源(实验预习指导)水硬度.doc
- 《分析化学》课程课后教学资源(实验预习指导)胃舒平中铝镁含量的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)COD的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)分光光度法测铁.doc
- 《分析化学》课程课后思考题(含答案)第一章.doc
- 《分析化学》课程课后思考题(含答案)第二章.doc
- 《分析化学》课程课后思考题(含答案)第三章.doc
- 《分析化学》课程课后思考题(含答案)第五章.doc
- 《分析化学》课程课后思考题(含答案)第六章.doc
- 《分析化学》课程课后思考题(含答案)第七章.doc
- 《分析化学》课程课后思考题(含答案)第八章.doc
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 1 The classification of analytical chemistry.ppt
- 《分析化学》课程教学资源(试题,含答案)分析化学试题10.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题9.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题8.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题7.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题6.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题5.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题4.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题3.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题2.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题1.doc
- 《分析化学》课程教学资源(知识拓展)自来水中余氯含量的测定.doc
- 《分析化学》课程教学资源(文献资料)一种快速简便测定奶粉中蛋白质的方法.pdf
- 《分析化学》课程教学资源(知识拓展)禁止化学武器组织获诺贝尔和平奖.doc
- 《分析化学》课程教学资源(知识拓展)碘盐中碘含量的测定.doc
- 《分析化学》课程教学资源(知识拓展)PM2.5的测定方法.doc
- 《分析化学》课程教学课件(PPT讲稿)沉淀滴定和重量分析.ppt
- 《分析化学》课程教学课件(PPT讲稿)氧化还原滴定法(Oxidation-Reduction Titration).ppt
- 《分析化学》课程教学课件(PPT讲稿)络合滴定.ppt
- 《分析化学》课程教学课件(PPT讲稿)酸碱滴定.ppt
