《分析化学》课程教学课件(PPT讲稿,英文)Chapter 3 summarization of Titrimetric analysis

©山本理大写 Analytical Chemistry Chapter 3 summarization of Titrimetric analysis Learning objectives To master the characteristics,classification of titrimetric analyis,the requirements for reactions used in titrimetric analysis; To master the methods to prepare standard solution,the determination and expression of the concentration;
Analytical Chemistry 2025/4/3 1 Chapter 3 summarization of Titrimetric analysis Learning objectives ◆To master the characteristics, classification of titrimetric analyis, the requirements for reactions used in titrimetric analysis; ◆To master the methods to prepare standard solution, the determination and expression of the concentration;

归东置2大写 Analytical Chemistry ◆To understand primary standard and the requirements for preparing the standard solution directly; to know about the difference between the stoichiometric point and the end point,four kinds of titration manner for titrimetric analysis
Analytical Chemistry 2025/4/3 2 ◆To understand primary standard and the requirements for preparing the standard solution directly; ◆ to know about the difference between the stoichiometric point and the end point, four kinds of titration manner for titrimetric analysis
G 归东理子大军 Analytical Chemistry 1.General principles Titrimetric analysis is one of the major divisions of analytical chemistry and the calculations involved are based on the simple stoichiometric relationships of chemical reactions. A titration is based on a chemical reaction that may be represented as: aA+bB→products
Analytical Chemistry 2025/4/3 3 1. General principles Titrimetric analysis is one of the major divisions of analytical chemistry and the calculations involved are based on the simple stoichiometric relationships of chemical reactions. A titration is based on a chemical reaction that may be represented as: aA +bB→products
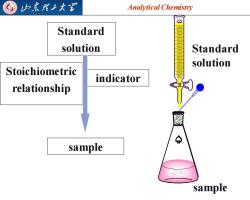
归东理工大军 Analytical Chemistry Standard solution Standard solution Stoichiometric indicator relationship sample sample
Analytical Chemistry 2025/4/3 4 Standard solution Stoichiometric relationship indicator sample Standard solution sample
山东理王大军 Analytical Chemistry 2.Terms of titrimetric analysis (1)Titrimetry The method to determinate the concentration of a sample by employing volumetric instruments and indicator. (2)Standard solution In titrimetric analysis,a reagent added as a solution of known concentration is referred to as a standard sulution
Analytical Chemistry 2025/4/3 5 2. Terms of titrimetric analysis (1) Titrimetry The method to determinate the concentration of a sample by employing volumetric instruments and indicator. (2) Standard solution In titrimetric analysis, a reagent added as a solution of known concentration is referred to as a standard sulution

归东觅子大图 Analytical Chemistry (3)Stoichiometric piont The point at which the titrant added just completely reacts with the analyte. (4)Titration curve The curve to describe the relationship between the titrant volume and the solution parameter during the process of the titration. (5)Indicator
Analytical Chemistry 2025/4/3 6 (3) Stoichiometric piont The point at which the titrant added just completely reacts with the analyte. (4) Titration curve The curve to describe the relationship between the titrant volume and the solution parameter during the process of the titration. (5) Indicator

山东理工大军 Analytical Chemistry The material used to indicate the end point because of the color change near the stoichiometric point. (6)End point The point at which the color of the indicator changes and the titration is stopped. The point is not necessarily equal to the stoichiometric point
Analytical Chemistry 2025/4/3 7 The material used to indicate the end point because of the color change near the stoichiometric point. (6) End point The point at which the color of the indicator changes and the titration is stopped. The point is not necessarily equal to the stoichiometric point

归东理工大军 Analytical Chemistry (7)Titration error The difference between the end point and the equivalence point. (8)Primary standard A S suitable,definite composition and highly pure material,used to determine the accurate concentration of standard solution. The requirements for a Primary standard:
Analytical Chemistry 2025/4/3 8 (7) Titration error The difference between the end point and the equivalence point. (8) Primary standard A suitable, definite composition and highly pure material, used to determine the accurate concentration of standard solution. The requirements for a Primary standard:

东理工大军 Analytical Chemistry The material should be of known composition and highly pure,99.9%pure,or better; It should undergo a rapid and stoichiometric chemical reaction with the solution being standardized; The material should stay stable indefinitely at room temperature and should withstand drying in an oven without change,it should not absorb water or carbon dioxide from the atomsphere;
Analytical Chemistry 2025/4/3 9 The material should be of known composition and highly pure, 99.9% pure, or better; It should undergo a rapid and stoichiometric chemical reaction with the solution being standardized; The material should stay stable indefinitely at room temperature and should withstand drying in an oven without change, it should not absorb water or carbon dioxide from the atomsphere;
归东置子大图 Analytical Chemistry It should,if possible,have a high equivalent weight (9)standardization The titration procedures of determining the concentration of standard solution with primary standard is called standardization. 3.Requirements of titrimetric analyis for chemical reaction
Analytical Chemistry 2025/4/3 10 (9) standardization It should, if possible, have a high equivalent weight The titration procedures of determining the concentration of standard solution with primary standard is called standardization. 3. Requirements of titrimetric analyis for chemical reaction
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《分析化学》课程教学资源(课件讲稿,英文)Chapter 4.pdf
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 5 Complexometric Titration.ppt
- 《分析化学》课程教学资源(课件讲稿,英文)Chapter 6 Oxidation-reduction titration.pdf
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 7 precipitation titration.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 8 visible spectrophotometry.ppt
- 《分析化学》课程课后教学资源(实验预习指导)天平.doc
- 《分析化学》课程课后教学资源(实验预习指导)滴定分析练习.doc
- 《分析化学》课程课后教学资源(实验预习指导)有机酸摩尔质量.doc
- 《分析化学》课程课后教学资源(实验预习指导)水硬度.doc
- 《分析化学》课程课后教学资源(实验预习指导)胃舒平中铝镁含量的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)COD的测定.doc
- 《分析化学》课程课后教学资源(实验预习指导)分光光度法测铁.doc
- 《分析化学》课程课后思考题(含答案)第一章.doc
- 《分析化学》课程课后思考题(含答案)第二章.doc
- 《分析化学》课程课后思考题(含答案)第三章.doc
- 《分析化学》课程课后思考题(含答案)第五章.doc
- 《分析化学》课程课后思考题(含答案)第六章.doc
- 《分析化学》课程课后思考题(含答案)第七章.doc
- 《分析化学》课程课后思考题(含答案)第八章.doc
- 《分析化学》课程课后思考题(含答案)第十章.doc
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 2 Errors and data treatment in quantitative analysis.ppt
- 《分析化学》课程教学课件(PPT讲稿,英文)Chapter 1 The classification of analytical chemistry.ppt
- 《分析化学》课程教学资源(试题,含答案)分析化学试题10.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题9.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题8.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题7.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题6.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题5.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题4.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题3.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题2.doc
- 《分析化学》课程教学资源(试题,含答案)分析化学试题1.doc
- 《分析化学》课程教学资源(知识拓展)自来水中余氯含量的测定.doc
- 《分析化学》课程教学资源(文献资料)一种快速简便测定奶粉中蛋白质的方法.pdf
- 《分析化学》课程教学资源(知识拓展)禁止化学武器组织获诺贝尔和平奖.doc
- 《分析化学》课程教学资源(知识拓展)碘盐中碘含量的测定.doc
- 《分析化学》课程教学资源(知识拓展)PM2.5的测定方法.doc
- 《分析化学》课程教学课件(PPT讲稿)沉淀滴定和重量分析.ppt
- 《分析化学》课程教学课件(PPT讲稿)氧化还原滴定法(Oxidation-Reduction Titration).ppt
- 《分析化学》课程教学课件(PPT讲稿)络合滴定.ppt
