电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 04 Cathode material for LIBs(LiFePO4)

Lecture 4 1956 Cathode material for LIBs: LiFePO, Chen Junsong School of Materials and Energy 2020.04
Cathode material for LIBs: LiFePO4 Chen Junsong School of Materials and Energy 2020.04 Lecture 4

Content /986 Discovery ● Chemistry of LiFePO4 Molecular structure and Li diffusion Advantages and disadvantages Synthesis and modification methods One literature example 2
Content • Discovery • Chemistry of LiFePO4 • Molecular structure and Li diffusion • Advantages and disadvantages • Synthesis and modification methods • One literature example 2

Discovery Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries A.K.Padhi,'K.S.Nanjundaswamy,"and J.B.Goodenough Center for Materials Science and Engineering,The University of Texas at Austin,Austin,Texas 78712-1063,USA Reversible extraction of lithium from LiFePO,and insertion of lithium into FePO at 3.5 V vs.lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low-power,rechargeable lithium battery that is inexpensive,nontoxic, Akshaya Padhi J.B.Goodenough and environmentally benign. J.Electrochem.Soc.,Vol.144,No.4,April 1997 3
Discovery Akshaya Padhi J. B. Goodenough J. Electrochem. Soc., Vol. 144, No. 4, April 1997 Reversible extraction of lithium from LiFePO4 and insertion of lithium into FePO4 at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low-power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. 3

Molecular structure of LiFePO4 FeOs octahedron PO,tetrahedron· The LiFePO4 olivine structure is formed by a hexagonal close packed oxygen array with Li+and Fe2+ occupying half of the octahedral sites and phosphorus occupying 1/8 of the tetrahedral sites in between the layers. Each FeO6 octahedron is corner linked to other FeO6 octahedra to Fe2+/3+ form zig-zag planes running parallel to the c-axis within alternating a-c D5+ planes. Li计 In addition,one edge of the 02 FeOs octahedron is shared with one PO4 tetrahedron and two edges are shared with two LiOs octahedra. The LiO octahedra form linear chains of edge shared octahedra between layers of FeOs octahedra and share edges with two PO4 tetrahedra https://www.iycr2014.org/learn/crystallography365/articles/20140429 4
FeO6 octahedron PO4 tetrahedron https://www.iycr2014.org/learn/crystallography365/articles/20140429 Molecular structure of LiFePO4 • The LiFePO4 olivine structure is formed by a hexagonal close packed oxygen array with Li+ and Fe2+ occupying half of the octahedral sites and phosphorus occupying 1/8 of the tetrahedral sites in between the layers. • Each FeO6 octahedron is corner linked to other FeO6 octahedra to form zig-zag planes running parallel to the c-axis within alternating a-c planes. • In addition, one edge of the FeO6 octahedron is shared with one PO4 tetrahedron and two edges are shared with two LiO6 octahedra. • The LiO6 octahedra form linear chains of edge shared octahedra between layers of FeO6 octahedra and share edges with two PO4 tetrahedra 4

Li diffusion in LiFePO4 795 Li PO FeOe -Curved 0.8 ----Linear 0.6 0.4 0.2 E0s o 0 0.1 02 03 0.4 0.5 a Migration coordinate https://openi.nlm.nih.gov/imgs/512/396/4285883/PMC428 Chem.Mater.,Vol.17,No.20,2005 5883_m-02-00085-fig1.png Activation energy of Li+ Li+travels preferably along the [010] migration in LiFePO,shows direction,and follows a curved that it is much lower if Lit pathway,jumping through adjacent follows a Curved than a tetrahedral and octahedral voids. Linear path. 5
Li diffusion in LiFePO4 b a Li PO4 FeO6 Li+ travels preferably along the [010] direction, and follows a curved pathway, jumping through adjacent tetrahedral and octahedral voids. Activation energy of Li+ migration in LiFePO4 shows that it is much lower if Li+ follows a Curved than a Linear path. https://openi.nlm.nih.gov/imgs/512/396/4285883/PMC428 5883_m-02-00085-fig1.png Chem. Mater., Vol. 17, No. 20, 2005 5
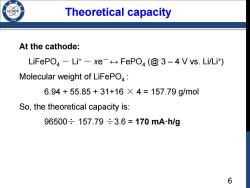
Theoretical capacity At the cathode: LiFePO-Li+-xe FePO(@3-4 V vs.Li/Li+) Molecular weight of LiFePO: 6.94+55.85+31+16×4=157.79g/mol So,the theoretical capacity is: 96500÷157.79÷3.6=170 mA.h/g 6
Theoretical capacity At the cathode: LiFePO4 - Li+ - xe- ↔ FePO4 (@ 3 – 4 V vs. Li/Li+) Molecular weight of LiFePO4 : 6.94 + 55.85 + 31+16 × 4 = 157.79 g/mol So, the theoretical capacity is: 96500÷ 157.79 ÷3.6 = 170 mA·h/g 6
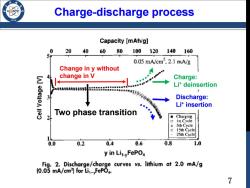
Charge-discharge process /986 Capacity [mAh/g] 0 2040 60 80 100 120 140 160 0.05mA/cm2,2.1mA/g Change in y without change in V Charge: Li*deinsertion 555188538553月 3 p靠年单e●a口的生学校共济、 Discharge: Lit insertion Two phase transition 2 Charging Ist Cycle Sth Cycle 0 15th Cycle 25th Cycle 0.0 0.2 0.4 0.6 0.8 1.0 y in Lit-yFePO Fig.2.Discharge/charge curves vs.lithium at 2.0 mA/g (0.05 mA/cm]for Li:-FePO4. 7
Charge-discharge process Change in y without change in V Discharge: Li+ insertion Charge: Li+ deinsertion Two phase transition 7
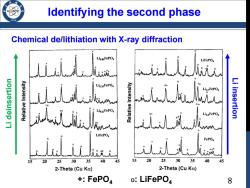
ldentifying the second l phase Chemical de/lithiation with X-ray diffraction Lio.osFePO4 人 LiFePO uolesulep !7 Lio.62FePO Li insertion Lio3FePO4 wM LIFePO i乱k 15 20 25 30 35 40 45 20 25 30 35 40 45 2-Theta(Cu Ko) 2-Theta(Cu Ka) +FePO4 o:LiFePO4 8
Chemical de/lithiation with X-ray diffraction Identifying the second phase Li deinsertion Li insertion +: FePO4 O: LiFePO4 8
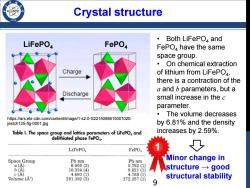
Crystal structure /98 Both LiFePO and LiFePO4 FePO4 FePO,have the same space group. On chemical extraction Charge of lithium from LiFePO4, there is a contraction of the a and b parameters,but a Discharge small increase in the c parameter. ·The volume decreases https://ars.els-cdn.com/content/image/1-s2.0-S2215098615001020- jestch125-fig-0001.jpg by 6.81%and the density Table I.The space group and lattice parameters of LiFePO and increases by 2.59%. delithiated phase FePO. LiFePO, FePO, Space Group Pbnm Pb nm Minor change in a(A】 6.008(3) 5.792(1) b(A) 10.334(4) 9.821(1) structuregood c(A) 4.693(1) 4.788(1) Volume (A) 291.392(3) 272.357(1) structural stability
LiFePO4 FePO4 Crystal structure • Both LiFePO4 and FePO4 have the same space group. • On chemical extraction of lithium from LiFePO4, there is a contraction of the a and b parameters, but a small increase in the c parameter. • The volume decreases by 6.81% and the density increases by 2.59%. Minor change in structure → good structural stability https://ars.els-cdn.com/content/image/1-s2.0-S2215098615001020- jestch125-fig-0001.jpg 1 9

Thermostability 9 TGA DSC 1002 65 N (a) No significant weight change 100.0 98 TGA 55 (Mw) up to 350 C 99.6 45 。 994 DSC A weight loss of ~1.6%when 2 35 90 reaching 500 C 25 988 Very little difference in TGA 6 15 98. curves in O2 9%2 100150200250300350 400 450500 No appreciable change in XRD TEMPERATURE (C) 100.2 (b) 100.0 TGA 2 998 9.6 94 High thermostability → 99.2 ~1.6% LHOI3M 99.0 High operating temperature 988 8.6 8.4 s0100150 200250300350400450500550 10 TEMPERATURE (C)
Thermostability N2 O2 ~1.6% • No significant weight change up to 350 oC • A weight loss of ~1.6% when reaching 500 oC • Very little difference in TGA curves in O2 • No appreciable change in XRD High thermostability → High operating temperature 2 10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 03 Lithium-ion Batteries(LiCoO).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 02 Introduction of Lithium Batteries.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 01 Background of Batteries(陈俊松).pdf
- “十四五”可再生能源发展规划(发布稿).pdf
- 《电力系统运行与控制 Power System Operation and Control》课程参考书籍文献:《Operation and Control in Power Systems》PDF电子书(Prof. P. S. R. MURTY).pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 10 Power System Security.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 09 Power System Optimal Power Flow.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 08 power system state estimation.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 07 Power System Wide-area Measurement and Control.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 06 Power System Reactive Power and Voltage Control.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 05 Power Generation Control and Frequency Regulation.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 04 Unit Commitment in Power System.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 03 Power System Economic Dispatch.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 02 Introduction.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 01 Introduction of the course(黄琦).pdf
- 银川能源学院(银川大学):《电力系统分析》课程教学资源(课件讲稿)第9章 电力系统稳定性分析.pdf
- 银川能源学院(银川大学):《电力系统分析》课程教学资源(课件讲稿)第8章 电力系统不对称故障分析.pdf
- 银川能源学院(银川大学):《电力系统分析》课程教学资源(课件讲稿)第7章 电力系统不对称运行分析方法.pdf
- 银川能源学院(银川大学):《电力系统分析》课程教学资源(课件讲稿)第6章 电力系统三相短路故障分析.pdf
- 银川能源学院(银川大学):《电力系统分析》课程教学资源(课件讲稿)第5章 电力系统功率平衡与控制.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 05 Cathode material for LIBs(Li-Mn-O and NCM).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 06 Anode material for LIBs(Graphite).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 07 Anode material for LIBs(Lithium).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section I Background and Fuel Cell(陈俊松).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section II Nuclear energy(Fundamentals of Fusion Enery).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section III Fundamentals of Solar Cell.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section IV THERMODYNAMICS.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 10 Anode material for LIB(TiO2).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 11 Safety of Li-ion Batteries.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 12 Solid-state Electrolyte in Li-ion Batteries(SSE of LIB).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 08 Anode Material for LIBs(Silicon).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 09 Anode material for LIBs(Tin).pdf
- 《电力系统自动化》:利用储能系统提升电网电能质量研究综述.pdf
- 《电力电子技术 Power Electronics》:电能质量指标及其算法的研究.pdf
- 深圳市标准化指导性技术文件:分布式光伏发电系统并网接入技术规范(SZDB/Z 227 - 2017)Technical specification for distributed photovoltaic generation system Grid-connected.pdf
- 智能电网:改善低压农网电压质量的分布式光伏——储能系统优化配置方法.pdf
- 国投甘肃小三峡发电有限公司:浅析水电厂AVC控制策略.pdf
- 中国电机工程学会:核能发电专业发展报告(PPT宣讲稿,2019年11月).pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第一章 绪论 1.1 传热学的研究内容及其在科学技术和工程中的应用 1.2 热能传递的三种基本方式.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第二章 稳态热传导 2.1 导热基本定律-傅里叶定律.pdf
