电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 09 Anode material for LIBs(Tin)

Lecture 9 196 Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04
Anode material for LIBs: Tin Chen Junsong School of Materials and Energy 2020.04 Lecture 9

Content /986 Introduction of Tin 。 Chemistry of Tin Molecular structure and Li insertion Synthesis and modification methods One literature example 2
2 Content • Introduction of Tin • Chemistry of Tin • Molecular structure and Li insertion • Synthesis and modification methods • One literature example
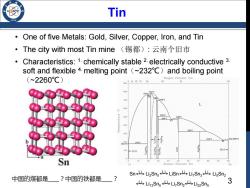
Tin One of five Metals:Gold,Silver,Copper,Iron,and Tin ·The city with most Tin mine(锡都):云南个旧市 Characteristics:1.chemically stable 2.electrically conductive 3. soft and flexible 4.melting point (~232C)and boiling point (~2260℃) Weight Percent Tin 9009406070 95 765 700 698C L 500 50BC 469c 486℃ 326 222C9 231.9081℃ 179G 10D (Sn)- Sn 40 60 100 Li Atomic Percent Tin Sn Sn<4Li2Sn,山LiSn<山Li,Sn3iLi5Snz 中国的煤都是?中国的铁都是 ? 山Li3Sne5 Lis Li,Sn2山Li2zSns 3
3 Tin Sn Li2Sn5 LiSn Li7Sn3 Li5Sn2 Li13Sn5 Li7Sn2 Li22Sn5 Li Li Li Li Li Li Li • One of five Metals: Gold, Silver, Copper, Iron, and Tin • The city with most Tin mine (锡都): 云南个旧市 • Characteristics: 1. chemically stable 2. electrically conductive 3. soft and flexible 4. melting point(~232℃)and boiling point (~2260℃)
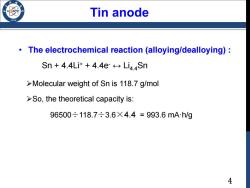
Tin anode /986 The electrochemical reaction (alloying/dealloying): Sn 4.4Li++4.4e Li4Sn >Molecular weight of Sn is 118.7 g/mol >So,the theoretical capacity is: 96500÷118.7÷3.6X4.4=993.6mAh/g 4
4 • The electrochemical reaction (alloying/dealloying) : Sn + 4.4Li+ + 4.4e- ↔ Li4.4Sn Molecular weight of Sn is 118.7 g/mol So, the theoretical capacity is: 96500÷118.7÷3.6×4.4 = 993.6 mA·h/g Tin anode
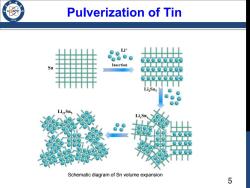
Pulverization of Tin /96 L Sn Insertion Li2Sns Liz2Sns Li Sn Schematic diagram of Sn volume expansion 5
5 Pulverization of Tin Schematic diagram of Sn volume expansion

Tin anode 196 Materials c Sn Density(gcm-3) 2.25 7.29 Lithiated phase LiCe Li44Sn Theoretical specific capacity(mAh g-1) 372 994 Theoretical charge density(mAh cm-3) 837 7246 Volume change (% 12 260 Potential vs.Li(~V) 0.05 0.6 Advantages Disadvantage high theoretical huge volume capacity change 中 D environmental pulverization of benignity the electrode 中 low cost poor cycle ability 6
6 Tin anode high theoretical capacity environmental benignity Advantages Disadvantage huge volume change pulverization of the electrode low cost poor cycle ability
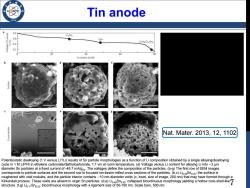
Tin anode 96 a 0.8 Li,Sns 入sn LiSn n/.n Li,Sna/Liz2Sns 0 20 40 60 Li content (at.%) apejins Nat.Mater..2013,12,1102 Potentiostatic dealloying (1 V versus Li/Li)results of Sn particle morphologies as a function of Li composition obtained by a single alloying/dealloying cycle in 1 M LiPF6 in ethylene carbonate/diethylcarbonate,1:1 v/v at room temperature.(a)Voltage versus Li content for alloying Li into ~2 um diameter Sn particles at a fixed current of-49.7 mA/gsn.The voltages define the composition of the particles.(b-g)The first row of SEM images corresponds to particle surfaces and the second row to focused ion-beam milled cross sections of the particles.(b,c)Li30Sn.70:the surface is roughened with void nodules,and the particle interior contains ~10 nm-diameter voids(c,inset,size of image,200 nm)that may have formed through a Kirkendall process.These voids are absent in virgin Sn particles.(d.e)Li4Sn52:collapsed bicontinuous morphology yielding a hollow core-shell-like7 structure.(f.g)Lio.Sn23:bicontinuous morphology with a ligament size of 50-100 nm.Scale bars,500 nm
7 Tin anode Potentiostatic dealloying (1 V versus Li+ /Li) results of Sn particle morphologies as a function of Li composition obtained by a single alloying/dealloying cycle in 1 M LiPF6 in ethylene carbonate/diethylcarbonate, 1:1 v/v at room temperature. (a) Voltage versus Li content for alloying Li into ∼2 μm diameter Sn particles at a fixed current of -49.7 mA/gSn. The voltages define the composition of the particles. (b-g) The first row of SEM images corresponds to particle surfaces and the second row to focused ion-beam milled cross sections of the particles. (b,c) Li0.30Sn0.70; the surface is roughened with void nodules, and the particle interior contains ∼10 nm-diameter voids (c, inset, size of image, 200 nm) that may have formed through a Kirkendall process. These voids are absent in virgin Sn particles. (d,e) Li0.48Sn0.52; collapsed bicontinuous morphology yielding a hollow core-shell-like structure. (f,g) Li0.77Sn0.23; bicontinuous morphology with a ligament size of 50-100 nm. Scale bars, 500 nm Nat. Mater. 2013, 12, 1102

Tin anode /98 ·Solution: >Nanosizing nanosphere nanocube nanowire nanotube .. >Coating Sn@C(good electronic conductivity,prevent aggregation, accommodate the strain of volume change)Sn@PPy .. >Alloying Sn-Cu Sn-Ni Sn-Sb Sn-Ni-Cu .. 8
8 Tin anode • Solution: Nanosizing nanosphere nanocube nanowire nanotube … Coating Sn@C(good electronic conductivity, prevent aggregation, accommodate the strain of volume change) Sn@PPy … Alloying Sn-Cu Sn-Ni Sn-Sb Sn-Ni-Cu …

A case study 思 D0:10.1002/adma.200701364 Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Spheres for High-Performance Anode Material in Lithium-Ion Batteries** By Wei-Ming Zhang,Jin-Song Hu,Yu-Guo Guo,Shu-Fa Zheng,Liang-Shu Zhong, Wei-Guo Song,and Li-Jun Wan* 24wt%20 Nanostructured tin dispersed in a carbon matrix In the present work,we therefore designed a new approach to synthesize tin nanoparticles encapsulated elastic hollow carbon and carbon-encapsulated hollow tin nanopartides were also spheres (TNHCs)with uniform size,in which multiple tin reported as superior anode materials.These studies showed nanoparticles with a diameter of less than 100 nm were encap- that both coating tin nanomaterials with carbon layer and sulated in one thin hollow carbonsphere with a thickness ofonly dispersing tin nanoparticles in carbon matrix are effective to about 20 nm,thus leading to both the content of Sn up to over improve their electrochemical properties in lithium ion batter-70%by weight and the void volume in carbon shell as high as ies.It is obvious that the higher content of and smaller size of tin,about 70-80%by volume.This void volume and the elasticity of as well as the thinner carbon coating will greatly contribute to thin carbon spherical shell efficiently accommodate the volume the further enhancement of material performance since the change of tin nanoparticles due to the Li-Sn alloying-dealloying lithium storage density in tin is much higher than that in carbon.reactions,and thus prevent the pulverization of electrode.As a Meanwhile,this tin-based anode material has to be designed to result,this type of tin-based nanocomposites have very high own enough void volume to compensate the volume expansion specific capacity of>800 mA h g in the initial 10 cycles,and during Lit insertion,which is important to improve its cycle >550mA hgafter the 100th cycle,as well as excellent cycling performance. y
9 A case study
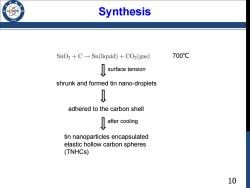
Synthesis 196 SnO2+C-Sn(liquid)+CO2(gas) 700℃ surface tension shrunk and formed tin nano-droplets 0 adhered to the carbon shell ater cooling tin nanoparticles encapsulated elastic hollow carbon spheres (TNHCs) 10
10 Synthesis 700℃ shrunk and formed tin nano-droplets adhered to the carbon shell after cooling tin nanoparticles encapsulated elastic hollow carbon spheres (TNHCs) surface tension
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 08 Anode Material for LIBs(Silicon).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 12 Solid-state Electrolyte in Li-ion Batteries(SSE of LIB).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 11 Safety of Li-ion Batteries.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 10 Anode material for LIB(TiO2).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section IV THERMODYNAMICS.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section III Fundamentals of Solar Cell.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section II Nuclear energy(Fundamentals of Fusion Enery).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第一部分)Section I Background and Fuel Cell(陈俊松).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 07 Anode material for LIBs(Lithium).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 06 Anode material for LIBs(Graphite).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 05 Cathode material for LIBs(Li-Mn-O and NCM).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 04 Cathode material for LIBs(LiFePO4).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 03 Lithium-ion Batteries(LiCoO).pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 02 Introduction of Lithium Batteries.pdf
- 电子科技大学:《物理与化学电源基础 Fundamental of Physical and Chemical Power Sources》课程教学资源(课件讲稿,第二部分)Lecture 01 Background of Batteries(陈俊松).pdf
- “十四五”可再生能源发展规划(发布稿).pdf
- 《电力系统运行与控制 Power System Operation and Control》课程参考书籍文献:《Operation and Control in Power Systems》PDF电子书(Prof. P. S. R. MURTY).pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 10 Power System Security.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 09 Power System Optimal Power Flow.pdf
- 电子科技大学:《电力系统运行与控制 Power System Operation and Control》课程教学资源(课件讲稿)Lecture 08 power system state estimation.pdf
- 《电力系统自动化》:利用储能系统提升电网电能质量研究综述.pdf
- 《电力电子技术 Power Electronics》:电能质量指标及其算法的研究.pdf
- 深圳市标准化指导性技术文件:分布式光伏发电系统并网接入技术规范(SZDB/Z 227 - 2017)Technical specification for distributed photovoltaic generation system Grid-connected.pdf
- 智能电网:改善低压农网电压质量的分布式光伏——储能系统优化配置方法.pdf
- 国投甘肃小三峡发电有限公司:浅析水电厂AVC控制策略.pdf
- 中国电机工程学会:核能发电专业发展报告(PPT宣讲稿,2019年11月).pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第一章 绪论 1.1 传热学的研究内容及其在科学技术和工程中的应用 1.2 热能传递的三种基本方式.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第二章 稳态热传导 2.1 导热基本定律-傅里叶定律.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第二讲 第一章 绪论 1.3 传热过程和传热系数 1.4 传热学的发展简史和研究方法.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第二章 稳态热传导 2.2 导热问题的数学描写 2.3 典型一维导热问题的分析解-通过平壁的导热.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第二章 稳态热传导 2.3 典型一维导热问题的分析解-通过圆筒壁的导热 2.4 通过肋片的导热.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第三章 非稳态热传导 3.1 非稳态导热的基本概念.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第三章 非稳态热传导 3.2 零维问题的分析法-集中参数法.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第四章 热传导问题的数值解法 4.1 导热问题数值求解的基本思想 4.2 内节点离散方程的建立方法.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第三章 非稳态热传导 3.3 典型一维非稳态导热的分析解.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第四章 热传导问题的数值解法 4.3 边界节点离散方程的建立及代数方程的求解.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第四章 热传导问题的数值解法 4.4 非稳态导热问题的数值解法.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第五章 对流传热的理论基础 5.1 对流传热概说 5.2 对流传热问题的数学描写.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第五章 对流传热的理论基础 5.3 边界层型对流传热问题的数学描写.pdf
- 山西能源学院:《传热学》课程教学资源(电子教案)第五章 对流传热的理论基础 5.4 流体外掠平板传热层流分析解及比拟理论.pdf
