厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 5 The structure of polyatomic molecules
Chapter 5 The structure of polyatomic molecules (A)
Chapter 5 The structure of polyatomic molecules (A)

5.1 Theory of hybrid orbital and atomic orbital hybridization 1.Hybrid orbital theory---Proposed by Pauling in 1928 Chemical bonding theories MOLECULAR ORBITAL THEORY Valence electrons are delocalized Valence electrons spread over entire molecule VALENCE BOND THEORY Valence electrons are localized between atoms (or are lone pairs). Half-filled atomic orbitals overlap to form bonds
MOLECULAR ORBITAL THEORY • Valence electrons are delocalized • Valence electrons spread over entire molecule. Chemical bonding theories VALENCE BOND THEORY • Valence electrons are localized between atoms (or are lone pairs). • Half-filled atomic orbitals overlap to form bonds. §5.1 Theory of hybrid orbital and atomic orbital hybridization 1. Hybrid orbital theory ---Proposed by Pauling in 1928
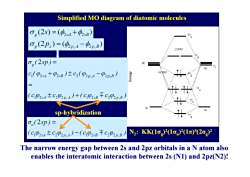
Simplified MO diagram of diatomic molecules Og(2S)=(44+42B) n9 0g(2p)=(4p.A-4p,B) LUMO Og(2sp)= 20 9 C(p2s4+p2sB)±C2(02p.A-02p.B) HOMO (C024±C292p,4)+(C102sB干C292p.B) sp-hybridization 3 ,(2sp)= (C924±C202p4)-(C102,B干C92p,B) N2:KK(1oe)21o)(1π)(2o) The narrow energy gap between 2s and 2pz orbitals in a N atom also enables the interatomic interaction between 2s(N1)and 2pz(N2)!
The narrow energy gap between 2s and 2pz orbitals in a N atom also enables the interatomic interaction between 2s (N1) and 2pz(N2)!

6e(2p)= C1(92s4+P2sB)±C2(p2:4+P2p.B) (C102s4±C22p,A)+(C92sB±C202p,B Boundary surfaces of (a)2s+2p (C4s4+C20p.4) (b)2s-2p (C04-C24p.4) Hybridization of 2s and 2p
Hybridization of 2s and 2p

Central Themes of Valence Bond Theory Basic Principle of Valence Bond Theory:a covalent bond forms when the orbitals from two atoms overlap and a pair of electrons occupies the region between the nuclei. 1)Opposing spins of the electron pair. 2)Maximum overlap of bonding orbitals. 3)Hybridization of atomic orbitals. Pauling proposed that the valence atomic orbitals in the molecule are different from those in the isolated atoms.We call this Hybridization!
Central Themes of Valence Bond Theory 1) Opposing spins of the electron pair. 2) Maximum overlap of bonding orbitals. 3) Hybridization of atomic orbitals. Pauling proposed that the valence atomic orbitals in the molecule are different from those in the isolated atoms. We call this Hybridization! Basic Principle of Valence Bond Theory: a covalent bond forms when the orbitals from two atoms overlap and a pair of electrons occupies the region between the nuclei
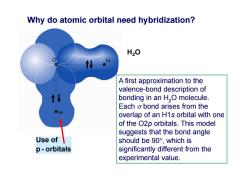
Why do atomic orbital need hybridization? H20 A first approximation to the valence-bond description of bonding in an H,O molecule. Each o bond arises from the overlap of an H1s orbital with one of the O2p orbitals.This model suggests that the bond angle Use of should be90°,which is p-orbitals significantly different from the experimental value
Use of p - orbitals H2O Why do atomic orbital need hybridization? A first approximation to the valence-bond description of bonding in an H2O molecule. Each bond arises from the overlap of an H1s orbital with one of the O2p orbitals. This model suggests that the bond angle should be 90, which is significantly different from the experimental value
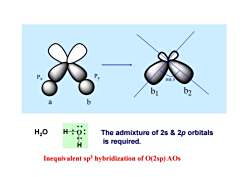
104.5 b1 b2 a H20 H:03 The admixture of 2s 2p orbitals H is required. Inequivalent sp3 hybridization of O(2sp)AOs
Px Py a b 104.5 b1 b2 H O H H2O The admixture of 2s & 2p orbitals is required. Inequivalent sp3 hybridization of O(2sp) AOs
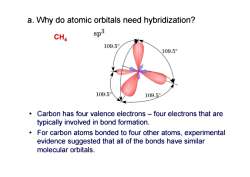
a.Why do atomic orbitals need hybridization? CH4 $p3 109.5 109.5° 109.5 109.5 Carbon has four valence electrons-four electrons that are typically involved in bond formation. For carbon atoms bonded to four other atoms,experimental evidence suggested that all of the bonds have similar molecular orbitals
• Carbon has four valence electrons – four electrons that are typically involved in bond formation. • For carbon atoms bonded to four other atoms, experimental evidence suggested that all of the bonds have similar molecular orbitals. CH 4 a. Why do atomic orbitals need hybridization?
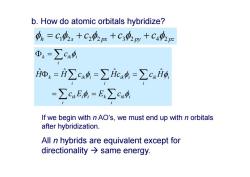
b.How do atomic orbitals hybridize? 4。=C2,+C202m+C3py+C44p ④k=∑c4 i=i∑cx=∑ic4=∑cxi0 =∑cxE,4,=E:∑cx中 If we begin with n AO's,we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy
b. How do atomic orbitals hybridize? i i i k ik i ik i i i i ik i i ik i k ik i i k ik c E E c H H c Hc c H c ˆ ˆ ˆ ˆ h s px py pz c c c c 1 2 22 3 2 42 If we begin with n AO’s, we must end up with n orbitals after hybridization. All n hybrids are equivalent except for directionality same energy

2.Construction of hybrid orbitals
2. Construction of hybrid orbitals
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 4 The structure of diatomic molecules.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 3 Molecular symmetry and symmetry point group.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 2 Atomic Structure.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 1 The Basic Knowledge of Quantum Mechanics(讲授:曹泽星、蒋亚琪).pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 9 多原子的半经验方法 Semi-experimental method for polyatomic molecular.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 8 共轭分子的结构与性能 Ab initio technique for polyatomic molecular.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 7 简单分子轨道理论 Elementary molecular orbital(MO)theory.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 6 分子的对称性与对称群 Molecular symmetry and symmetric group.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 5 原子结构 Atomic structure.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 4 角动量与自旋 Angle momentum and spin.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 3 矩阵与算符 Matrix and operator.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 2 简单量子力学体系 Rudimental quantum mechanics system.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 1 Schrödinger 方程 Schrodinger equation.ppt
- 中国科学技术大学:《高分子化学》课程教学资源(课件讲稿)第六、七、八、九章 离子聚合、开环聚合、链式共聚合反应、配位聚合、聚合物的化学反应(主讲:刘世勇).pdf
- 和频光谱在生物膜等生物界面中的应用 In situ molecular level studies on membrane related peptides and proteins in real time using sum frequency generation vibrational spectroscopy.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十二章 镧系元素和锕系元素.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十一章 过渡元素(二).pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十章 过渡元素(一).pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十八章 铜、锌副族.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十七章 碱金属与碱土金属.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 6 Polyatomic molecules(II).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Group Theory and Its Application to Quantum Chemistry - Introduction(主讲:曹泽星).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Part I 群论基础 Chapter 1 基本概念 Chapter 2 抽象群的结构.pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 3 群的类分解 Chapter 4 商群与同态(Factor group & Homomorphism)Chapter 5 群的直积(Direct Product)Chapter 6 置换群(Permutation group/Symmetric group).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 7 Cayley定理 Chapter 8 线性向量空间(Linear vector spaces)Chapter 9 线性算符(Linear Operator)Chapter 10 群的表示.pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 11 酉空间(Unitary Space)Chapter 12 表示的约化及其判据 Chapter 13 正交定理 The Orthogonality Relations Chapter 14 直积群的表示 Chapter 15 表示的分解 Chapter 16 投影算符(Projection Operator).pdf
- 《普通化学》课程电子教案(PPT教学课件)第9章 仪器分析基础.pptx
- 山西师范大学:《生物化学》课程教学大纲.pdf
- 山西师范大学:《生物化学实验》课程教学大纲.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)绪论(主讲:任浩).pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第四章 分子结构.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第一章 量子力学基础.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第二章 运动的量子理论.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第六章 固体.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第三章 原子结构和原子光谱.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第五章 对称性.pdf
- 安徽建筑大学:材料与化学工程学院应用化学专业培养方案(2019版).doc
- 安徽建筑大学:材料与化学工程学院《高分子化学实验》课程教学大纲.pdf
- 山东农业大学:《生物化学实验B》课程教学大纲.pdf
- 山东农业大学:《生物化学实验A》课程教学大纲.pdf
