厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 7 简单分子轨道理论 Elementary molecular orbital(MO)theory

Chapter 7 Electronic Structure of Diatomic Molecules 7.1 The Hydrogen Molecule Ion 1.Accurate solutions a 0 R b The H2*molecule.The nuclei are at a and b;R is the internuclear distance;roand ro are the distances from the the electron to nuclei a and 6 In the BO approximation,the Hamiltonian in atomic units is =-12-1-1+1 2 a店R Since the nuclei are fixed,1/R is a constant.The purely electronic Hamiltionian for H2can be rewritten as He1= 12 11 Ta Ih (7.1) Obviously,the Schroedinger equation is not separable in spherical polar coordinates.In 1927,Burrau showed that the separation of variables is possible using confocal elliptic coordinates.n.. g a The elliptic coordinates with two centers
Chapter 7 Electronic Structure of Diatomic Molecules 7.1 The Hydrogen Molecule Ion 1. Accurate solutions . . a b O R -e φ r ra rb The H2 + molecule. The nuclei are at a and b; R is the internuclear distance; ra and rb are the distances from the the electron to nuclei a and b. In the BO approximation, the Hamiltonian in atomic units is r r R H a b 1 1 1 2 1 2 = − ∇ − − + ∧ Since the nuclei are fixed, 1/R is a constant. The purely electronic Hamiltionian for H2 + can be rewritten as a b el r r H 1 1 2 1 2 = − ∇ − − ∧ (7.1) Obviously, the Schroedinger equation is not separable in spherical polar coordinates. In 1927, Burrau showed that the separation of variables is possible using confocal elliptic coordinates ξ,η,ϕ. . . a R b ra rb The elliptic coordinates with two centers ϕ

5=名+名 7=名6 R R (7.2) The ranges of these coordinates are: 0≤p≤2π,1≤5≤0,-1≤n≤1 (7.3) We have =2R(5+) 5=25-) (7.4) The H2*electronic Hamiltonian(7.1)in the elliptic coordinates has the form Hd=- 2 R2(E2-72) +2是+0-) -2 n 1102 2 2(7.5) -2&+g-11-7002RE+m)RE-》7 Hence the Schroedinger equation is HΨ(5,n,p)=EΨ(5,n,p) (7.6) Let Ψ(5,n,p)=L(5)M(n)Φ(p) (7.7) Substitution of(7.7)into the Schroedinger equation (7.6)leads to an equation in which the variables are separable d2 D(p)=-m2D(o) do" (7.8) m2 (g-224+25+,Rg-2L=079
R r r R r r a b a − b = + ξ = , η (7.2) The ranges of these coordinates are: 0 ≤ ϕ ≤ 2π , 1 ≤ ξ ≤ ∞, −1 ≤ η ≤ 1 (7.3) We have ( ) 2 1 ( ), 2 1 ra = R ξ +η rb = R ξ −η (7.4) The H2 + electronic Hamiltonian (7.1) in the elliptic coordinates has the form ( ) 2 ( ) 2 ) ] 1 1 1 1 2 ( [( 1) 2 (1 ) ( ) 2 2 2 2 2 2 2 2 2 2 2 2 2 2 η ξ η ϕ ξ η ξ η η η η ξ ξ ξ ξ ξ η − − + − ∂ ∂ − + − + ∂ ∂ − ∂ ∂ + − ∂ ∂ + ∂ ∂ − − = − ∧ R R R Hel (7.5) Hence the Schroedinger equation is Ψ(ξ,η,ϕ) = Ψ(ξ,η,ϕ) ∧ el H el E (7.6) Let Ψ(ξ,η,ϕ) = L(ξ )M (η)Φ(ϕ) (7.7) Substitution of (7.7) into the Schroedinger equation (7.6) leads to an equation in which the variables are separable. ( ) ( ) 2 2 2 ϕ ϕ ϕ Φ = −m Φ d d (7.8) ) 0 2 1 1 ( 1) ' ' 2 ' ( 2 2 2 2 2 2 = − − + + + + − L m L L A R EelR ξ ξ ξ ξ ξ (7.9)

()M-2M-(4RM0 10 Solving the equation(7.8),we have (p)= V2π (7.11) where m=0,±l,+2,.. [1 when m=0 √2π (p)= -cosmo V2 when m 1,2... (7.12) √2 -sin mo The solution for M(n)is an infinite series of associated Legendre functions.L()also involves an infinite series.The requirement that the wave function be well behaved leads to the conclusion that for a fixed value of R,only certain values of Ee are allowed (i.e.discrete);this gives a series of electronic states,corresponding electronic energy E(R)=E+ R (7.13) The electronic energies must be calculated numerically.The series of energy levels are marked by quantum numbers n,I,and m,whose allowed values are n=1,2,3,… l≤n-1 (7.14) m≤I 10,1,2,3,… s,p,d,f
) 0 2 1 1 (1 ) ' ' 2 ' ( 2 2 2 2 2 = − − − − + + M m M M A EelR η η η η (7.10) Solving the equation (7.8), we have ϕ π ϕ im e 2 1 Φ( ) = (7.11) where m = 0, ±1, ±2, … or = Φ = ϕ π ϕ π π ϕ m m when m sin 2 1 cos 2 1 0 2 1 ( ) when m = 1, 2, … (7.12) The solution for M(η) is an infinite series of associated Legendre functions. L(ξ) also involves an infinite series. The requirement that the wave function be well behaved leads to the conclusion that for a fixed value of R, only certain values of Eel are allowed (i.e. discrete); this gives a series of electronic states, corresponding electronic energy R E R Eel 1 ( ) = + (7.13) The electronic energies must be calculated numerically. The series of energy levels are marked by quantum numbers n, l, and m, whose allowed values are m l l n n ≤ ≤ − = 1 1, 2, 3, " (7.14) l 0, 1, 2, 3, … s, p, d, f. …
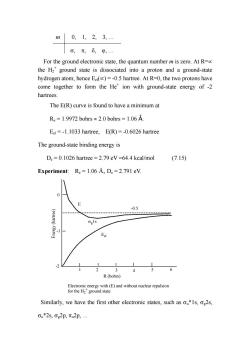
m0,1,2,3, o,元,80,… For the ground electronic state,the quantum number m is zero.At R= the H2*ground state is dissociated into a proton and a ground-state hydrogen atom;hence Eer(oc)=-0.5 hartree.At R=0,the two protons have come together to form the He*ion with ground-state energy of-2 hartrees. The E(R)curve is found to have a minimum at Re=1.9972 bohrs 2.0 bohrs 1.06 A. Eel=-1.1033 hartree,E(R)=-0.6026 hartree The ground-state binding energy is D.=0.1026 hartree =2.79 eV=64.4 kcal/mol (7.15) Experiment:Re=1.06 A,De=2.791 eV. 0.5 Ggls E 3 456 R(bohrs) Electronic energy with(E)and without nuclear repulsion for the H2 ground state Similarly,we have the first other electronic states,such as s,o2s. ou*2s,cg2p,元u2p
m 0, 1, 2, 3, … σ, π, δ, ϕ, … For the ground electronic state, the quantum number m is zero. At R=∝ the H2 + ground state is dissociated into a proton and a ground-state hydrogen atom; hence Eel(∝) = -0.5 hartree. At R=0, the two protons have come together to form the He+ ion with ground-state energy of -2 hartrees. The E(R) curve is found to have a minimum at Re = 1.9972 bohrs ≈ 2.0 bohrs = 1.06 Å. Eel = -1.1033 hartree, E(R) = -0.6026 hartree The ground-state binding energy is De = 0.1026 hartree = 2.79 eV =64.4 kcal/mol (7.15) Experiment: Re = 1.06 Å, De = 2.791 eV. 0 -1 -2 R (bohrs) 1 2 3 4 5 6 Eel E Electronic energy with (E) and without nuclear repulsion for the H2 + ground state σg1s -0.5 Similarly, we have the first other electronic states, such as σu*1s, σg2s, σu*2s, σg2p, πu2p, …

2.Approximate Treatment of the H2" Trial function: Consider a limiting behavior of the H2*ground-state electronic wave function as R approaches p=CalSa +CplSp (7.16) -e-n When R goes zero,we get the He'ion,which has the ground-state wave function(Z=2) 2 1\π (7.17) Note that at the R=0the trial function(7.16)goes to (G+c2) e V Comparing with (7.17),we see that (7.16)has the wrong limiting behavior at R =0.We can fix things by multiplying ra and ro in the exponentials by a variational parameter k=k(R).For correct limiting behavior at zero and infinite internuclear distance,we have K(0)=2,k(oc)=1 We thus take as the trial function 0=Ca功sa+C691sb (7.18) where k 4π -e-kn (7.19)
2. Approximate Treatment of the H2 + Trial function: Consider a limiting behavior of the H2 + ground-state electronic wave function as R approaches ∝. a a b b ϕ = c 1s + c 1s (7.16) a br b r a s e s e − − = = π π 1 , 1 1 1 When R goes zero, we get the He+ ion, which has the ground-state wave function (Z=2) r e 2 3 2 − π (7.17) Note that at the R = 0 the trial function (7.16) goes to r c c e− + π 1 ( ) 1 2 . Comparing with (7.17), we see that (7.16) has the wrong limiting behavior at R = 0. We can fix things by multiplying ra and rb in the exponentials by a variational parameter k = k(R). For correct limiting behavior at zero and infinite internuclear distance, we have K(0) = 2, k(∝) =1 We thus take as the trial function a sa b sb c c ϕ = φ1 + φ 1 (7.18) where a b kr sb kr sa e k e k − − = = π φ π φ 3 1 3 1 , (7.19)

The molecular orbital (MO)(7.18)is a linear combination of atomic orbitals,an LCAO-MO.(Exponential parameter-effective nuclear charge) For the trial function(7.18),the secular equation is H-WS.H-WS=0 Hba-WSba Hob-WSpb (7.20) The integrals Haa and Hob are Hnm=∫4nH4ndk,Hb=∫4ibH4dy(I.21)) These two integrals are called Coulomb integrals.Since the H2'is a homonuclear diatomic system,Han=Hob.We have Hb=∫gaH4bdy,Ha=∫4bH4dy(7.22) Since is Hermitian and the functions in these integrals are real,we conclude that Hab=Hpa.The integral Hab,is called a resonance (or bond) integral.Sinceand are normalized and real.We have Sa==pi中1adh=1=Sb Sab=∫gin中i6dr=Sba (7.23) The secular equation(7.20)becomes H-W Ha-WS.=0 Hab -WSab Hoo-W (7.24) Hoo -W=(Hob-SabW) (7.25) 所-月tH4,所-二H 1+Sab 1-Sh (7.26) These two roots are upper bounds for the energies of the ground and the first excited electronic states of H2
The molecular orbital (MO) (7.18) is a linear combination of atomic orbitals, an LCAO-MO. (Exponential parameter—effective nuclear charge) For the trial function (7.18), the secular equation is = 0 − − − − ba ba bb bb aa aa ab ab H WS H WS H WS H WS (7.20) The integrals Haa and Hbb are Haa φ sa Hφ sadν Hbb φ sb Hφ1sbdν * 1 1 * 1 , ∫ ∫ ∧ ∧ = = (7.21) These two integrals are called Coulomb integrals. Since the H2 + is a homonuclear diatomic system, Haa = Hbb. We have Hab φ sa Hφ sbdν Hba φ sb Hφ1sadν * 1 1 * 1 , ∫ ∫ ∧ ∧ = = (7.22) Since ∧ H is Hermitian and the functions in these integrals are real, we conclude that Hab = Hba. The integral Hab is called a resonance (or bond) integral. Since φ1sa and φ1sb are normalized and real. We have ab sa sb ba aa sa sa sa sa bb S dv S S dv S = = == = = ∫ ∫ 1 * 1 1 * 1 | 1 1 1 φ φ φ φ φ φ (7.23) The secular equation (7.20) becomes = 0 − − − − H WS H W H W H WS ab ab aa aa ab ab (7.24) H W (H S W ) aa − = ± ab − ab (7.25) ab aa ab ab aa ab S H H W S H H W − − = + + = 1 , 1 1 2 (7.26) These two roots are upper bounds for the energies of the ground and the first excited electronic states of H2 +

We now find the coefficients in (7.18)for each of the roots of the secular equation.From the linear homogeneous equations in Chapter 5, we have (H-W)c+(Hh-S)c=0 (7.27 Substituting in W from(7.26),we get Ca=l Co (7.28) =Ca(Osa +oisb) (7.29) We fix ca by normalization: cP∫(2++244h)d=1 Ico (7.30) 2+28 The normalized trial function corresponding to the energy W is thus: 1 01= =(9a+中b) V2+2S (7.31) For the root W2,we find c=-co and 1 0=2-2 =(4La-1b) (7.32) Let us evaluate Haa,Hab,and Sab Overlap integral Sat Sw=e1+R+片2R] (7.34) Coulomb integral Haa (7.35) Resonance integral Hab
We now find the coefficients in (7.18) for each of the roots of the secular equation. From the linear homogeneous equations in Chapter 5, we have ( − ) + ( − ) = 0 aa a ab ab b H W c H S c (7.27) Substituting in W1 from (7.26), we get = 1 b a c c (7.28) ( ) 1 a 1sa 1sb ϕ = c φ +φ (7.29) We fix ca by normalization: ab a a sa sb sa sb S c c dv 2 2 1 | | | | | ( 2 ) 1 1 1 2 1 2 1 2 1 1 + = = + + ⋅ = ∫ ϕ ϕ φ φ φ φ (7.30) The normalized trial function corresponding to the energy W1 is thus: ( ) 2 2 1 1 1sa 1sb ab S ϕ φ + φ + = (7.31) For the root W2, we find cb = -ca and ( ) 2 2 1 2 1sa 1sb ab S ϕ φ −φ − = (7.32) Let us evaluate Haa, Hab, and Sab. Overlap integral Sab ] 3 1 [1 2 2 S e kR k R kR ab = + + − (7.34) Coulomb integral Haa ) 1 ( 1 2 1 2 2 R e k R H k k kR aa = − − + + − (7.35) Resonance integral Hab

H=号25+6k-2+Re" (7.36) The final task is to vary the parameter k,setting =0,a亚=0 k ak The results are that for the (Is+1sp)function(7.31),the parameter k increases monotonically from I to 2 as R decreases from to 0;for the 2(1s -1sp)function(7.32),k decreases monotonically from 1.0 to 0.4 as R decreases from to 0.Since k is positive and never greater than 2,and since the overlap integral Sab is positive,we see from (7.36)that the resonance integral Hb is always negative. The ground state o1s m=H。+H幽 1 1+Sab 01= V2+2S =(91a+41b) k(R)=1.24 Re=2.02 bohrs,Binding energy:2.35 eV.(Expt.2.00 bohrs,2.79 eV). If we omit varying k,but simply set it equal to 1,we get Re=2.50 bohrs,Binding energy:1.76 eV The excited state ou 1s m=H。-Hb V2-254.4) 1 1-Sab The appearance of the trial functions for og Is and ouls
kR ab ab H k S k k kR e− = − + ( − 2)(1+ ) 2 1 2 (7.36) The final task is to vary the parameter k, setting 0, 0 1 2 = ∂ ∂ = ∂ ∂ k W k W The results are that for the ϕ1 (1sa +1sb) function (7.31), the parameter k increases monotonically from 1 to 2 as R decreases from ∝ to 0; for the ϕ2 (1sa -1sb) function (7.32), k decreases monotonically from 1.0 to 0.4 as R decreases from ∝ to 0. Since k is positive and never greater than 2, and since the overlap integral Sab is positive, we see from (7.36) that the resonance integral Hab is always negative. The ground state σg 1s : ab aa ab S H H W + + = 1 1 , ( ) 2 2 1 1 1sa 1sb ab S ϕ φ + φ + = k(Re) = 1.24 Re = 2.02 bohrs, Binding energy: 2.35 eV. (Expt. 2.00 bohrs, 2.79 eV). If we omit varying k, but simply set it equal to 1, we get Re = 2.50 bohrs, Binding energy: 1.76 eV. The excited state σu 1s : ab aa ab S H H W − − = 1 2 , ( ) 2 2 1 2 1sa 1sb ab S ϕ φ −φ − = The appearance of the trial functions for σg 1s and σu 1s

Charge probability density In summary,we have formed the two H2*molecular orbitals(MO),one bonding and one antibonding,from the atomic orbitals Isa and Isp.The MO energy are given by(7.26)as 所2=Hn±h-AS 1±Sb (7.37) A schematic representation of MO formation from AO's Nr(中a-b) N(仲a+b)
. . . . a b a b . . a b . . a b 1sa 1 2 2 1sb 1 2 2 z z z z ϕ1 2 ϕ2 2 Charge probability density In summary, we have formed the two H2 + molecular orbitals (MO), one bonding and one antibonding, from the atomic orbitals 1sa and 1sb. The MO energy are given by (7.26) as ab ab aa ab aa S H H S W H ± − = ± 1 1,2 (7.37) A schematic representation of MO formation from AO’s N'(φa − φb) N(φa + φb) φa φb

Trial function from hybridized AO's(Dickinson in 1933) p=[1s。+c(2p)a]+[1s6+c(2po)b] (7.38) where c is variational parameter,and where 4a=\π (2p)(2p.)cos0. Final results: K=1.246,ξ=2.965,c=0.138 R.=2.01 bohrs,Binding energy:2.73 eV. 3.Molecular Orbitals for H2*Excited States Method of the linear variation function LCAO-MO's C Isa +C22sa +C3(2po)caISs +Cs25p +Co(2po) The symmetry of the homonuclear diatomic molecule makes the coefficients of the atom b orbitals equal to 1 times the corresponding atom a orbital coefficients: p=[G1s。+c22s。+c3(2Po)a]±[G1s6+C22s6+C3(2p)b](7.39) where the upper sign goes with the even(g)states(i.e.+:g;-:u). Relative magnitudes of the coefficients in(7.39) For the electronic states that dissociate into a Is hydrogen atom:Large c Small c2 and c3.As a first approximation,we set c2 and c3 equal to zero, taking
Trial function from hybridized AO’s (Dickinson in 1933) [1 (2 ) ] [1 (2 ) ] a 0 a b p0 b ϕ = s + c p + s + c (7.38) where c is variational parameter, and where a a r a z a kr sa e p p e r k a a θ π ξ π φ ξ cos 4(2 ) , (2 ) (2 ) / 2 1/ 2 5/ 2 0 3 1 − − = = = Final results: K = 1.246, ξ =2.965, c = 0.138 Re = 2.01 bohrs, Binding energy: 2.73 eV. 3. Molecular Orbitals for H2 + Excited States Method of the linear variation function LCAO-MO’s a a a b b p b c 1s c 2s c (2 p ) c 1s c 2s c (2 ) ϕ = 1 + 2 + 3 0 + 4 + 5 + 6 0 The symmetry of the homonuclear diatomic molecule makes the coefficients of the atom b orbitals equal to ±1 times the corresponding atom a orbital coefficients: [ 1 2 (2 ) ] [ 1 2 (2 ) ] 1 a 2 a 3 0 a 1 b 2 b 3 p0 b ϕ = c s + c s + c p ± c s + c s + c (7.39) where the upper sign goes with the even (g) states (i.e. +: g; -: u). Relative magnitudes of the coefficients in (7.39) For the electronic states that dissociate into a 1s hydrogen atom: Large c1, Small c2 and c3. As a first approximation, we set c2 and c3 equal to zero, taking
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 6 分子的对称性与对称群 Molecular symmetry and symmetric group.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 5 原子结构 Atomic structure.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 4 角动量与自旋 Angle momentum and spin.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 3 矩阵与算符 Matrix and operator.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 2 简单量子力学体系 Rudimental quantum mechanics system.ppt
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 1 Schrödinger 方程 Schrodinger equation.ppt
- 中国科学技术大学:《高分子化学》课程教学资源(课件讲稿)第六、七、八、九章 离子聚合、开环聚合、链式共聚合反应、配位聚合、聚合物的化学反应(主讲:刘世勇).pdf
- 和频光谱在生物膜等生物界面中的应用 In situ molecular level studies on membrane related peptides and proteins in real time using sum frequency generation vibrational spectroscopy.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十二章 镧系元素和锕系元素.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十一章 过渡元素(二).pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第二十章 过渡元素(一).pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十八章 铜、锌副族.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十七章 碱金属与碱土金属.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十四章 氮族元素.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十五章 碳族元素(碳、硅、硼).pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十二章 卤素.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十三章 氧族元素.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十一章 氧化还原反应.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第十章 电解质溶液.pdf
- 上饶师范学院:《无机化学》课程电子教案(课件讲稿)第八章 化学平衡.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 8 共轭分子的结构与性能 Ab initio technique for polyatomic molecular.pdf
- 厦门大学:《量子化学 Quantum Chemistry》课程电子教案(教学课件)Chapter 9 多原子的半经验方法 Semi-experimental method for polyatomic molecular.ppt
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 1 The Basic Knowledge of Quantum Mechanics(讲授:曹泽星、蒋亚琪).pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 2 Atomic Structure.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 3 Molecular symmetry and symmetry point group.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 4 The structure of diatomic molecules.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 5 The structure of polyatomic molecules.pdf
- 厦门大学:《结构化学 Structural Chemistry》课程教学资源(课件讲稿)Chapter 6 Polyatomic molecules(II).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Group Theory and Its Application to Quantum Chemistry - Introduction(主讲:曹泽星).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Part I 群论基础 Chapter 1 基本概念 Chapter 2 抽象群的结构.pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 3 群的类分解 Chapter 4 商群与同态(Factor group & Homomorphism)Chapter 5 群的直积(Direct Product)Chapter 6 置换群(Permutation group/Symmetric group).pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 7 Cayley定理 Chapter 8 线性向量空间(Linear vector spaces)Chapter 9 线性算符(Linear Operator)Chapter 10 群的表示.pdf
- 厦门大学:《群论及其在化学中的应用》课程教学资源(课件讲稿)Chapter 11 酉空间(Unitary Space)Chapter 12 表示的约化及其判据 Chapter 13 正交定理 The Orthogonality Relations Chapter 14 直积群的表示 Chapter 15 表示的分解 Chapter 16 投影算符(Projection Operator).pdf
- 《普通化学》课程电子教案(PPT教学课件)第9章 仪器分析基础.pptx
- 山西师范大学:《生物化学》课程教学大纲.pdf
- 山西师范大学:《生物化学实验》课程教学大纲.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)绪论(主讲:任浩).pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第四章 分子结构.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第一章 量子力学基础.pdf
- 中国石油大学(华东):《结构化学》课程电子教案(教学课件)第二章 运动的量子理论.pdf
