中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 4 药物的构效关系(SAR)
Chapter4.药物的构效关系(SAR) Structure-Activity Relationships -67973 674973 本章目录 L药物结构 Ⅱ.药物的作用靶点(Targets) Ⅲ药物-靶标的分子识别和相互作用 V定量构效关系
Chapter 4. 药物的构效关系(SAR) Structure-Activity Relationships 本章目录 I. 药物结构 II. 药物的作用靶点(Targets) III. 药物-靶标的分子识别和相互作用 IV. 定量构效关系
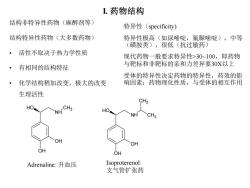
L药物结构 结构非特异性药物(麻醉剂等) 特异性(specificity) 结构特异性药物(大多数药物) 特异性极高(如尿嘧啶,氟脲嘧啶),中等 (磺胺类),很低(抗过敏药) 活性不取决于热力学性质 现代药物一般要求特异性>30~100,即药物 有相同的结构特征 与靶标和非靶标的亲和力差异要30X以上 受体的特异性决定药物的特异性,药效的影 化学结构稍加改变,极大的改变 响因素:药物理化性质,与受体的相互作用 生理活性 CH3 HO NH CH3 HO NH CH3 OH OH OH OH Adrenaline:升血压 Isoproterenol: 支气管扩张药
I. 药物结构 结构非特异性药物(麻醉剂等) 结构特异性药物(大多数药物) • 活性不取决于热力学性质 • 有相同的结构特征 • 化学结构稍加改变,极大的改变 生理活性 OH NH OH HO CH3 OH NH OH HO CH3 CH3 Adrenaline: 升血压 Isoproterenol: 支气管扩张药 特异性(specificity) 特异性极高(如尿嘧啶,氟脲嘧啶),中等 (磺胺类),很低(抗过敏药) 现代药物一般要求特异性>30~100,即药物 与靶标和非靶标的亲和力差异要30X以上 受体的特异性决定药物的特异性,药效的影 响因素:药物理化性质,与受体的相互作用

小分子药物结构解析 General structure elucidation procedure: 1.Determine molecular formula by MS 2.Degree of Unsaturation =[2+(2 x #Carbons)+#Nitrogens- #Hydrogens-#Halogens]/2 3.Acquire 1H,HSQC and HMBC,write down chemical shifts and build connectivity 4.Acquire 1D TOCSY NOESY to resolve ambiguity 5.Draw a tentative structure and check consistency with spectra Note:the presence of peaks can be proof of nuclear and its connectivity the absence of peaks can NOT be proof of no connectivity!
小分子药物结构解析 General structure elucidation procedure: 1. Determine molecular formula by MS 2. Degree of Unsaturation = [2 + (2 x #Carbons) + #Nitrogens - #Hydrogens - #Halogens]/2 3. Acquire 1H, HSQC and HMBC, write down chemical shifts and build connectivity 4. Acquire 1D TOCSY & NOESY to resolve ambiguity 5. Draw a tentative structure and check consistency with spectra Note: the presence of peaks can be proof of nuclear and its connectivity the absence of peaks can NOT be proof of no connectivity!
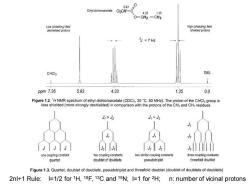
593 Ethyl dichloroacetate Cl2CH-0 4.33.1.35 O-CH2-CH3 Low(shielding)feld High (shielding)field deshielded protons shielded protons 为=7H2 CHCI TMS ppm 7.26 5.93 4.33 1.35 0.0 Figure 1.2.'H NMR spectrum of ethyl dichloroacetate(CDCla.25C.80 MHz).The proton of the CHCI2 group is less shielded(more strongly deshielded)in comparison with the protons of the CH2 and CH,residues ,> 12 3 one coupling constant two coupling constants two similar coupling constants three coupling constants quartet doublet of doublets pseudotriplet threefold doublet Figure 1.3.Quartet,doublet of doublets,pseudotriplet and threefold doublet(doublet of doublets of doublets) 2nl+1 Rule:I=1/2 for 1H,19F,13C and 15N;I=1 for 2H; n:number of vicinal protons
2nI+1 Rule: I=1/2 for 1H, 19F, 13C and 15N; I=1 for 2H; n: number of vicinal protons
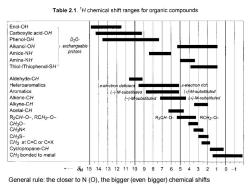
Table 2.1.'H chemical shift ranges for organic compounds Enol-OH Carboxylic acid-OH Phenol-OH D20- Alkanol-OH exchangeable Amide-NH protons Amine-NH Thiol-/Thiophenol-SH Aldehyde-CH Heteroaromatics π-electron deficient -electron rich Aromatics (-)-M-substituted /+月M-substit近uted Alkene-CH (-)-M-substituted (+)-M-substituted Alkyne-CH Acetal-CH R2CH-0-,RCH2-0- R2CH-0- RCH2-O- CH30- CHgN< CH3S- CH3 at C=C or C=X Cyclopropane-CH CH3 bonded to metal ·—61514131211109876543210 General rule:the closer to N(O),the bigger(even bigger)chemical shifts
General rule: the closer to N (O), the bigger (even bigger) chemical shifts
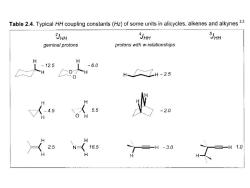
Table 2.4.Typical HH coupling constants(Hz)of some units in alicycles,alkenes and alkynes2 3JHH JHH JHH geminal protons protons with w-relationships H -12.5 -6.0 H H-2.5 4.5 5.5 -2.0 2.5 16.5 =-H-30 三一H 1.0 H
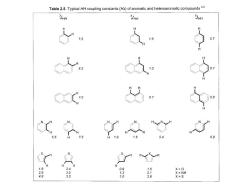
Table 2.5.Typical HH coupling constants(Hz)of aromatic and heteroaromatic compounds23 3JHH JHH 5JHH 7 0.7 0.9 H 1.8 3.4 0.9 X=0
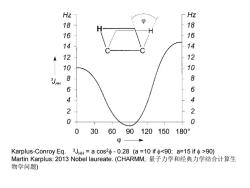
Hz Hz g 18 16 14 14 12 12 10 10 8 8 6 6 4 4 2 2 0 0 0 306090120150 180° p Karplus-Conroy Eq.3Jhh=acos2Φ-0.28(a=10ifΦ90) Martin Karplus:2013 Nobel laureate.(CHARMM,量子力学和经典力学结合计算生 物学问题)
Karplus-Conroy Eq. 3JHH = a cos2f - 0.28 (a =10 if f90) Martin Karplus: 2013 Nobel laureate. (CHARMM, 量子力学和经典力学结合计算生 物学问题)

Table 2.2.1C chemical shift ranges for organic compounds 200 150 O(TMS) Carbenlum-lons Ketones Aldehydes Acetals.Ketals Quinones Carboxylic acids and derivs. Thioureas Ureas.Carbonates Oximes Imines Cyanides Thiocyanates tsocyanates Cyanates Carbodimides Heteroaromatics Aromatics (Cyclo-)Alkenes -1-M-sub (Cyclo-)Alkynes (Cyclo-)Alkanes R3C-0- R3C-NR2 RaC-S- R3C-Halogen R2CH-0- R2CH-NR2 R2CH-S- R2CH-Haloger H3C-0 HgC-NR2 H3C-S- H3C-Halogen Alkyi bonded to meta 200 150 100 O (TMS
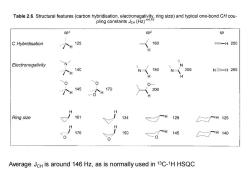
Tabl2.Structurau (hyiin)ad typical o-bond Sp3 sp2 sp C Hybridisation 125 160 三-H250 H Electronegativity N N 个H 140 N 180 N人 205 N=H269 0 0 "个H145 O个H 170 200 Ring size 161 134 129 个H125 176 150 145 H140 Average JcH is around 146 Hz,as is normally used in 13C-1H HSQC
Average JCH is around 146 Hz, as is normally used in 13C- 1H HSQC
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 3b 前体药物 Prodrug.ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 3a 药代动力学PKPD(DMPK).ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 2b 先导化合物优化 Lead Optimization.ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 2a Drug screening & discovery(design).ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 1 Introduction Medicinal Chemistry(授课教师:阮科).ppt
- 《物理化学》课程教学资源:物理化学 Physical Chemistry(简明版)各章习题与答案(第二版,共十章,天津大学物理化学教研室编).pdf
- 《物理化学》课程教学资源:物理化学 Physical Chemistry(简明版)教材PDF电子版(第二版,共十章,天津大学物理化学教研室编).pdf
- 清华大学出版社:《物理化学》课程书籍教材(上下册)PDF电子版(上下册共十二章,编著:朱文涛).pdf
- 广东工业大学:《物理化学》课程教学资源(练习题解)第十章 胶体化学.pdf
- 广东工业大学:《物理化学》课程教学资源(课件讲稿)第十章 胶体化学(PPT).ppt
- 广东工业大学:《物理化学》课程教学资源(课件讲稿)第九章 化学动力学(PPT).ppt
- 广东工业大学:《物理化学》课程教学资源(文献资料)外压法推导开尔文公式的探究.pdf
- 广东工业大学:《物理化学》课程教学资源(文献资料)表面过剩及吉布斯吸附等温式的探讨.pdf
- 广东工业大学:《物理化学》课程教学资源(练习题解)第八章 表面现象.pdf
- 广东工业大学:《物理化学》课程教学资源(课件讲稿)第八章 界面现象.pdf
- 广东工业大学:《物理化学》课程教学资源(文献资料)液体接界电势公式分析.pdf
- 广东工业大学:《物理化学》课程教学资源(练习题解)第七章 电化学.doc
- 广东工业大学:《物理化学》课程教学资源(课件讲稿)第七章 电化学(PPT).ppt
- 广东工业大学:《物理化学》课程教学资源(练习题解)第六章 相平衡.pdf
- 广东工业大学:《物理化学》课程教学资源(课件讲稿)第六章 相平衡.pdf
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 6 心血管药物 Cardiovascular Drugs.ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 5 癌症 Cancer(1/2).ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 7 作用神经系统的药物(中枢神经系统 CNS).ppt
- 中国科学技术大学:《药物化学》课程教学资源(PPT课件讲稿)Chapter 5 癌症 Cancer(2/2).ppt
- 《药物化学》课程文献资料(Medicinal Chemistry)Name of common cyclic compounds.docx
- 《药物化学》课程文献资料(Medicinal Chemistry)Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Prioritization of cancer therapeutic targets using CRISPR–Cas9 screens.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Structural Mechanism for Statin Inhibition of HMG-CoA Reductase.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Application of the Three-Dimensional Structures of Protein Target Molecules in Structure-Based Drug Design.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Epigenetic protein families - a new frontier for drug discovery.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)GLIVEC(STI571, IMATINIB),A RATIONALLY DEVELOPED,TARGETED ANTICANCER DRUG,Nat Rev Drug Disc 2002.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Personalized medicine in oncology - the future is now.pdf
- 《药物化学》课程文献资料(Medicinal Chemistry)Selective inhibition of BET bromodomains.pdf
- 三明学院:化学工程与工艺专业课程教学大纲汇编.pdf
- 三明学院:材料化学专业课程教学大纲汇编.pdf
- 三明学院:资源与化工学院材料化学专业课程教学大纲汇编.pdf
- 绍兴文理学院:化学化工学院应用化学专业课程教学大纲汇编.pdf
- 绍兴文理学院:化学化工学院化学专业课程教学大纲汇编.pdf
