上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 27-28_Applying 2nd law to thermodynamic cycles, Maximum performance

上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lectures 27-28 Chapter 6 The Second law of thermodynamics (Section 6.7-6.11) Spring,2017 强 Prof.,Dr.Yonghua HUANG 月是a http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lectures 27-28 Spring, 2017 Prof., Dr. Yonghua HUANG Chapter 6 The Second law of thermodynamics (Section 6.7 -6.11) http://cc.sjtu.edu.cn/G2S/site/thermo.html

Questions about Thermodynamic Cycles How much of the heat put in at high temperature can be converted to work? What is the maximum work? Can two engines working between the same two heat reservoirs drive one another? Nicolas Leonard Sadi Carnot 上游究通大学 Apr/5 Wed,2017 2 SHANGHAI JLAO TONG UNIVERSITY
Apr/5 Wed, 2017 2 Questions about Thermodynamic Cycles How much of the heat put in at high temperature can be converted to work? What is the maximum work? Can two engines working between the same two heat reservoirs drive one another? Nicolas Léonard Sadi Carnot
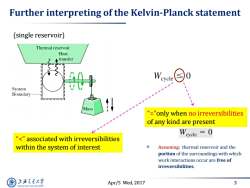
Further interpreting of the Kelvin-Planck statement (single reservoir) Thermal reservoir Heat Atransfer W ycle System Boundary Mass "="only when no irreversibilities of any kind are present "<"associated with irreversibilities within the system of interest Assuming:thermal reservoir and the portion of the surroundings with which work interactions occur are free of irreversibilities. 上游充通大 Apr/5 Wed,2017 3 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 3 Further interpreting of the Kelvin-Planck statement Assuming: thermal reservoir and the portion of the surroundings with which work interactions occur are free of irreversibilities. “<” associated with irreversibilities within the system of interest “=”only when no irreversibilities of any kind are present (single reservoir)
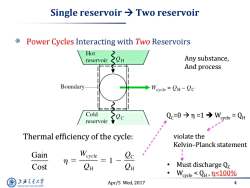
Single reservoir Two reservoir Power Cycles Interacting with Two Reservoirs Hot reservoir Any substance, And process Boundary ◆Weycle=QH-Qc Cold reservoir Qc=0今n=1→Wycle=QH Thermal efficiency of the cycle: violate the Kelvin-Planck statement Gain n= =1- Cost C CH Must discharge Qc Weycle<QH,n≤100% 上游充通大 Apr/5 Wed,2017 4 SHANGHAI JLAO TONG UNIVERSITY
Apr/5 Wed, 2017 4 Single reservoir Two reservoir Power Cycles Interacting with Two Reservoirs Thermal efficiency of the cycle: QC=0 η =1 Wcycle = QH violate the Kelvin–Planck statement • Must discharge QC • Wcycle < QH , η<100% Any substance, And process Gain Cost
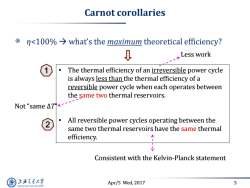
Carnot corollaries nwhat's the maximum theoretical efficiency? Less work The thermal efficiency of an irreversible power cycle is always less than the thermal efficiency of a reversible power cycle when each operates between the same two thermal reservoirs. Not“same△T<-- All reversible power cycles operating between the same two thermal reservoirs have the same thermal efficiency. Consistent with the Kelvin-Planck statement 上游充通大学 Apr/5 Wed,2017 5 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 5 Carnot corollaries η<100% what’s the maximum theoretical efficiency? • The thermal efficiency of an irreversible power cycle is always less than the thermal efficiency of a reversible power cycle when each operates between the same two thermal reservoirs. • All reversible power cycles operating between the same two thermal reservoirs have the same thermal efficiency. Not “same ΔT” Less work Consistent with the Kelvin-Planck statement 1 2
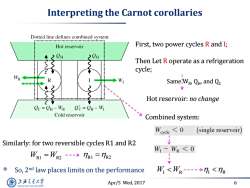
Interpreting the Carnot corollaries Dotted line defines combined system Hot reservoir First,two power cycles R and I; Then Let R operate as a refrigeration cycle; WR W SameW Q and Qc Hot reservoir:no change Oc=CH-WR Oc=CH-WI Cold reservoir Combined system: W cle 7R1=R2 So,2nd law places limits on the performance W<W-→1<R 上游充通大 Apr/5 Wed,2017 6 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 6 Interpreting the Carnot corollaries First, two power cycles R and I; Then Let R operate as a refrigeration cycle; Same WR , QH , and QC Hot reservoir: no change Combined system: W W I R I R W W R1 R2 R1 R2 Similarly: for two reversible cycles R1 and R2 So, 2nd law places limits on the performance
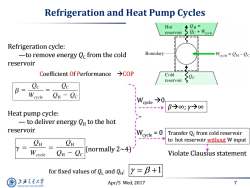
Refrigeration and Heat Pump Cycles Hot CH= reservoir c+Weyele Refrigeration cycle: -to remove energy Qc from the cold Boundary Weyele OH-Oc reservoir Coefficient Of Performance >COP Cold reservoir Qe B Weycle CH-Qc B→∞;Y→∞ Heat pump cycle: -to deliver energy OH to the hot reservoir cycle =0 Transfer Qc from cold reservoir CH to hot reservoir without W input Wcycle Cn-Qc (normally 2~4) Violate Clausius statement for fixed values of Q and Q: Y=B+1 上泽通大学 Apr/5 Wed,2017 7 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 7 Refrigeration and Heat Pump Cycles Refrigeration cycle: —to remove energy QC from the cold reservoir Heat pump cycle: — to deliver energy QH to the hot reservoir Coefficient Of Performance COP for fixed values of QL and QH : 1 (normally 2~4) Wcycle 0 β∞; γ∞ Wcycle = 0 Transfer QC from cold reservoir to hot reservoir without W input Violate Clausius statement
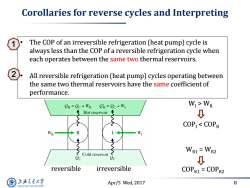
Corollaries for reverse cycles and Interpreting The COP of an irreversible refrigeration (heat pump)cycle is always less than the COP of a reversible refrigeration cycle when each operates between the same two thermal reservoirs. All reversible refrigeration (heat pump)cycles operating between the same two thermal reservoirs have the same coefficient of performance. CH=Oc+WR OH=Qc+WI WI>WR Hot reservoir 0 COPI<COPR WR R W WR1=WR2 2c Cold reservoir 2c ↓ reversible irreversible COPR1=COPR2 上游文通大学 Apr/5 Wed,2017 8 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 8 Corollaries for reverse cycles and Interpreting reversible irreversible WI > WR COPI < COPR WR1 = WR2 COPR1 = COPR2 • The COP of an irreversible refrigeration (heat pump) cycle is always less than the COP of a reversible refrigeration cycle when each operates between the same two thermal reservoirs. • All reversible refrigeration (heat pump) cycles operating between the same two thermal reservoirs have the same coefficient of performance. 1 2
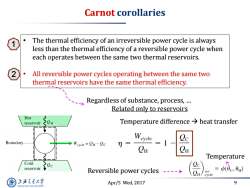
Carnot corollaries ● The thermal efficiency of an irreversible power cycle is always less than the thermal efficiency of a reversible power cycle when each operates between the same two thermal reservoirs. All reversible power cycles operating between the same two thermal reservoirs have the same thermal efficiency. Regardless of substance,process,... Related only to reservoirs Hot reservoir Temperature difference>heat transfer Boundary Weyele=CH-Oc m= CH CH Temperature Cold reservoir Reversible power cycles ---- rev cycle 上降文通大学 Apr/5 Wed,2017 9 SHANGHAI JIAO TONG UNIVERSITY
Apr/5 Wed, 2017 9 Carnot corollaries • The thermal efficiency of an irreversible power cycle is always less than the thermal efficiency of a reversible power cycle when each operates between the same two thermal reservoirs. • All reversible power cycles operating between the same two thermal reservoirs have the same thermal efficiency. Regardless of substance, process, … Related only to reservoirs Temperature difference heat transfer Reversible power cycles Temperature 1 2
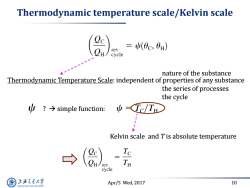
Thermodynamic temperature scale/Kelvin scale =(0c,0H) cycle nature of the substance Thermodynamic Temperature Scale:independent of properties of any substance the series of processes the cycle y?→simple function: ψ(Tc/TH Kelvin scale and Tis absolute temperature Te rey TH cycle 上游气通大粤 Apr/5 Wed,2017 10 SHANGHAI JLAO TONG UNIVERSITY
Apr/5 Wed, 2017 10 Thermodynamic temperature scale/Kelvin scale Thermodynamic Temperature Scale: independent of properties of any substance ? simple function: Kelvin scale and T is absolute temperature nature of the substance the series of processes the cycle
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 23-24_Introducing 2nd law, concept of irreversibilities.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 22_Transient analysis of Energy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 20-21_Illustrations_3 Heat exchangers, throttling devices, System integration.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 19_Illustrations_2 Compressors, pumps.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 18_Illustrations_1 Nozzles, diffusers, turbines.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 17_Control volume analysis - energy conservation.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 16_Control volume analysis - mass conservation.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 15_Polytropic process.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第五章 对流传热的理论基础 5-1 对流传热概说 5-2 对流换热问题的数学描写.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第四章 导热问题的数值解法 §4-4 非稳态导热问题的数值解法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第三章 非稳态导热 § 3-3 典型一维物体非稳态导热的分析解 §3-4 半无限大物体的非稳态导热 第四章 导热问题的数值解法 4-1 导热问题数值求解的基本思想 4-2 内节点离散方程的建立方法 4-3 边界结点离散方程的建立及代数方程的求解.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 2-5 具有内热源的一维导热问题 2-6 多维稳态导热的求解 第三章 非稳态导热 3-1 非稳态导热的基本概念 3-2 零维问题的分析法——集中参数法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 2-5 具有内热源的一维导热问题 2-6 多维稳态导热的求解 第三章 非稳态导热 3-1 非稳态导热的基本概念 3-2 零维问题的分析法——集中参数法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 §2-3 典型一维稳态导热问题的分析解 2-4 通过肋片的导热.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 2-1 导热基本定律 2-2 导热问题的数学描写.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第五章 对流传热的理论基础 5-3 边界层型对流传热问题的数学描写 5-4 流体外掠平板传热层流分析解及比拟理论 第六章 单相对流传热的实验关联式 6-1 相似原理与量纲分析.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)传热传质学 Heat and Mass Transfer 第一章 绪论(王平阳).pdf
- 上海交通大学:《能源物理》教学资源(课件讲义)第一章 质点运动学.pdf
- 上海中医药大学:《物理学》课程教学资源(PPT课件)第十四章 原子光谱与分子光谱.ppt
- 上海中医药大学:《物理学》课程教学资源(PPT课件)第十二章 光学基本知识与药用光学仪器.ppt
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 29_Carnot Cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 30_Clausius inequality and Entropy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 31_Retrieve entropy data.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 32_Internally reversible processes, Closed system entropy balance.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 33_Entropy increase principle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 34_Entropy balance to open systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 35_Isentropic processes, Isentropic efficiencies.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 36_Heat transfer and Work of internal reversible, ss flow.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 37-38_Concept of exergy and apply to CM&CV systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 39-40_vapor power cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 43_superheat and reaheat.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 44_Vapor-compression refrigeration, Heat pump systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 45_Air standard cycle, internal combustion engines, Otto cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 46_Diesel cycle and dual cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 47_Compressor, compression with intercooling.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 48_Review and Final Exam.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)中意楼位置.pptx
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 01-02_Course Introduction.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 03-04_Concepts.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 05-06_Energy, work, heat transfer.pdf
