上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 34_Entropy balance to open systems

上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 34 Chapter 7 Entropy (Section 7.13) Spring,2017 强 Prof.,Dr.Yonghua HUANG RAn员P http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 34 Spring, 2017 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.13) http://cc.sjtu.edu.cn/G2S/site/thermo.html

Entropy rate balance for CV systems Like mass and energy,transferred by streams Closed system +o T →open system special dSev dt ∑m空m rate of rates of rate of entropy entropy entropy change transfer production 上游究通大学 Wednesday,April 19,2017 2 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 2 Entropy rate balance for CV systems Like mass and energy, transferred by streams Closed system open system special

Integral form of entropy balance eq.for CV 号空包4 Heat flux time rate of entropy Sv(t) transfer accompanying heat transfer 0 引ar-1(》n+人w-pea)-i velocity 上游充通大 Wednesday,April 19,2017 3 SHANGHAI JIAO TONG UNIVERSITY
Wednesday, April 19, 2017 3 Integral form of entropy balance eq. for CV Heat flux velocity

CV at steady state Steady state: Mass balance: ∑m=∑m Energy balance: 0-a.-成,+区a(a+是+-Σe+号+s Entropy balance: 70 dS= "'dt 汽区 Not conserved!! These equations often must be solved simultaneously. irreversibilities 上游充通大 Wednesday,April 19,2017 4 SHANGHAI JIAO TONG UNIVERSITY
Wednesday, April 19, 2017 4 CV at steady state Steady state: Mass balance: Energy balance: Entropy balance: 0 These equations often must be solved simultaneously. Not conserved!! irreversibilities
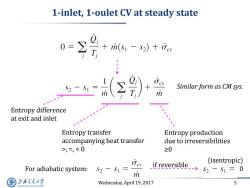
1-inlet,1-oulet CV at steady state 0= T ri(s1 -s2)+fev +r ( Similar form as CM sys. m Entropy difference at exit and inlet Entropy transfer Entropy production accompanying heat transfer due to irreversibilities >,=,52-S=0 m 上游充通大学 Wednesday,April 19,2017 5 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 5 1-inlet, 1-oulet CV at steady state Entropy transfer accompanying heat transfer >, =, < 0 Entropy production due to irreversibilities ≥0 Similar form as CM sys. For adiabatic system: if reversible 0 (isentropic) Entropy difference at exit and inlet
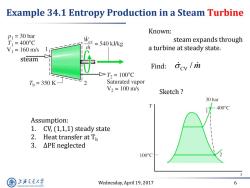
Example 34.1 Entropy Production in a Steam Turbine Known: P =30 bar T1=400°C Wey =540 kJ/kg steam expands through V1=160m/s a turbine at steady state. steam Find: Gev/m D>T2=100C Tb=350K- Saturated vapor V2=100m/s Sketch 30 bar 400°C Assumption: 1.CV,(1,1,1)steady state 2.Heat transfer at T 3.△PE neglected 100C 上游充通大学 Wednesday,April 19,2017 6 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 6 Example 34.1 Entropy Production in a Steam Turbine Find: steam Known: steam expands through a turbine at steady state. CV / m Assumption: 1. CV, (1,1,1) steady state 2. Heat transfer at Tb 3. ∆PE neglected Sketch ?
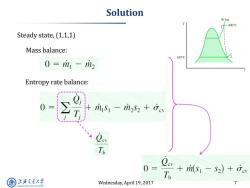
Solution 30 bar 400°C Steady state,(1,1,1) Mass balance: 100C 0=m1-m2 Entropy rate balance: ris-mi2s2 +ev To 0 sis)o To 上游究通大学 Wednesday,April 19,2017 7 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 7 Solution Steady state, (1,1,1) Mass balance: Entropy rate balance:

Solution cont. 30 bar 400°C Tp +(S1-S2)+0cv 100°C Q./m 个 (S2-S1) m Energy balance equation:∠? -+%+() i TabA-6,(30bar,400C)→h1=3230.9k/kg; TabA-4,(100C)→h2=hg=2676.1k/kg =540+(2676.1-3230.9 m kg +[r,u@(g引neo 1kJ =540-554.8-7.8=-22.6kJ/kg 上游充通大学 Wednesday,April 19,2017 8 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 8 Solution cont. Energy balance equation: ?? Tab A-6, (30bar, 400˚C)h1 = 3230.9 kJ/kg; Tab A-4, (100˚C)h2=hg = 2676.1 kJ/kg ??

Solution cont. 30bar 400°C TabA-4,(100C)→s2=7.3549k/kg TabA-6,(30bar,400°C→s1=6.9212k/kg-K; 100°C -22.6kJ/kg、 Qev/ri +(S2-S1) m Tp (-22.6kJkg) 350K +(7.3549-6.9212) = 0.0646+0.4337=0.4983kJ/kg·K 图 上游充通大 Wednesday,April 19,2017 9 SHANGHAI JIAO TONG UNIVERSITY
Wednesday, April 19, 2017 9 Solution cont. Tab A-6, (30bar, 400˚C)s1 = 6.9212kJ/kg-K; Tab A-4, (100˚C)s2=7.3549 kJ/kg

Example 34.2 Evaluating a performance claim a single stream of air An inventor claims: T1=21C P1=5 bars a device requiring no energy transfer by work or 2 Inlet heat transfer.Separate air T2=79C into hot and cold streams. P2=I bar Hot outlet Evaluate the inventor's claim, Cold outlet T3=-18C P3=I bar 60%mass Assumption: 1.Steady state 2.CV:Qcv=0,Wcv=0 3. ideal gas model for air,cp=1.0 kJ/kg-K 4.ignoring APE and AKE of the streams from inlet to exit. 上游充通大 Wednesday,April 19,2017 10 SHANGHAI JLAO TONG UNIVERSITY
Wednesday, April 19, 2017 10 Example 34.2 : Evaluating a performance claim An inventor claims: a device requiring no energy transfer by work or heat transfer. Separate air into hot and cold streams. Evaluate the inventor’s claim, a single stream of air 60% mass Assumption: 1. Steady state 2. CV: Qcv=0, Wcv=0 3. ideal gas model for air, cp=1.0 kJ/kg-K 4. ignoring ∆PE and ∆KE of the streams from inlet to exit
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 33_Entropy increase principle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 32_Internally reversible processes, Closed system entropy balance.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 31_Retrieve entropy data.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 30_Clausius inequality and Entropy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 29_Carnot Cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 27-28_Applying 2nd law to thermodynamic cycles, Maximum performance.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 23-24_Introducing 2nd law, concept of irreversibilities.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 22_Transient analysis of Energy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 20-21_Illustrations_3 Heat exchangers, throttling devices, System integration.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 19_Illustrations_2 Compressors, pumps.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 18_Illustrations_1 Nozzles, diffusers, turbines.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 17_Control volume analysis - energy conservation.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 16_Control volume analysis - mass conservation.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 15_Polytropic process.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第五章 对流传热的理论基础 5-1 对流传热概说 5-2 对流换热问题的数学描写.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第四章 导热问题的数值解法 §4-4 非稳态导热问题的数值解法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第三章 非稳态导热 § 3-3 典型一维物体非稳态导热的分析解 §3-4 半无限大物体的非稳态导热 第四章 导热问题的数值解法 4-1 导热问题数值求解的基本思想 4-2 内节点离散方程的建立方法 4-3 边界结点离散方程的建立及代数方程的求解.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 2-5 具有内热源的一维导热问题 2-6 多维稳态导热的求解 第三章 非稳态导热 3-1 非稳态导热的基本概念 3-2 零维问题的分析法——集中参数法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 2-5 具有内热源的一维导热问题 2-6 多维稳态导热的求解 第三章 非稳态导热 3-1 非稳态导热的基本概念 3-2 零维问题的分析法——集中参数法.pdf
- 上海交通大学:《传热学》课程教学资源(课件讲稿)第二章 导热基本定律及稳态导热 §2-3 典型一维稳态导热问题的分析解 2-4 通过肋片的导热.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 35_Isentropic processes, Isentropic efficiencies.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 36_Heat transfer and Work of internal reversible, ss flow.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 37-38_Concept of exergy and apply to CM&CV systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 39-40_vapor power cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 43_superheat and reaheat.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 44_Vapor-compression refrigeration, Heat pump systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 45_Air standard cycle, internal combustion engines, Otto cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 46_Diesel cycle and dual cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 47_Compressor, compression with intercooling.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 48_Review and Final Exam.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)中意楼位置.pptx
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 01-02_Course Introduction.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 03-04_Concepts.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 05-06_Energy, work, heat transfer.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 07-08_Energy balance for close system and cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 09-10_Substance, property and phase.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 11_Retrieving pvt properties.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 12_Evaluating u, h, cp, cv properties.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 13_Equation of state and ideal gas model.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 14_cv, cp, Δu, Δh of ideal gas and applied to close system.pdf
