中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十三章 含氮有机化合物 Nitrogen Containing Organic Compounds

Chapter 13Nitrogen Containing Organic Compounds
Chapter 13 Nitrogen Containing Organic Compounds

13.1 Nitro Compounds13.1.1 Nomenclature and PhysicPropertiesR-NO2RNORONORONO2NitriteNitrocompoundNitrateNitric compoundNomenclature:NO, as a substituted groupR
13.1 Nitro Compounds 13.1.1 Nomenclature and Physic Properties R-NO2 R O N O 2 R N O R O N O Nitro compound Nitrate Nitric compound Nitrite Nomenclature: NO2 as a substituted group R N O O π 3 4 R N O O

13.1.2 Preparation13.1.2.1 Gaseous nitraton of aliphatichydrocarbonsCH3CH,CHNOCH,CHCH3400℃CHCH,CH3+HONO2NO2CH3CHNO2CH3NO2
13.1.2 Preparation 13.1.2.1 Gaseous nitraton of aliphatic hydrocarbons 400℃ HONO2 CH3CH2CH2NO2 CH3CHCH3 NO2 CH3CH2NO2 CH3NO2 CH3CH2CH3 +

13.1.2.2 Nucleophilic substitution+CH3(CH2)6CH,NO2 +CH3(CH2)CH,ONOCH3(CH2)6CH,I+AgNO211%83%RCH,NO2CH2-RCH,ONO
13.1.2.2 Nucleophilic substitution 83% 11% CH3 (CH2 )6CH2I+ AgNO2 CH3 (CH2 )6CH2NO2 +CH3 (CH2 )6CH2ONO -O N O : + CH2 X R RCH2NO2 RCH2ONO + X O - :N O

13.1.2.3 Oxidation of tertiary aminesCH3CH3KMnO4CH3 --C一NH2CH-C-NO2CH3CH383%
13.1.2.3 Oxidation of tertiary amines CH3 C NH2 CH3 CH3 KMnO4 CH3 C NO2 CH3 CH3 83%
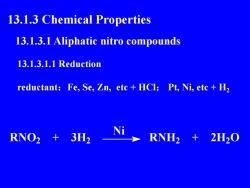
13.1.3 ChemicalProperties13.1.3.1 Aliphatic nitro compounds13.1.3.1.1 Reductionreductant: Fe, Se, Zn, etc +HCl; Pt, Ni, etc +H,NRNO23H2RNH22H20
13.1.3 Chemical Properties 13.1.3.1 Aliphatic nitro compounds 13.1.3.1.1 Reduction reductant:Fe, Se, Zn, etc + HCl; Pt, Ni, etc + H2 RNO2 + 3H2 Ni RNH2 + 2H2O

13.1.3.1.2 Acidity of a -HCH2=H+[CH2CH3-CH2O浴(假酸式)OHNaOHJCH 2=CHNa0(酸式)
13.1.3.1.2 Acidity of α-H (假酸式) (酸式) CH2 N OH O - [CH 2 N + O - O - ] Na NaOH + CH3 N O O - + H + + [ -CH2 N O -CH2 N O O O CH2= +N O - O -] - -
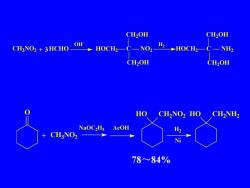
CH,OHCH,OHOHHNO2CH3NO2+3HCHOHOCH,-HOCH2NH2CH,OHCH,OHHOCH,NO2HOCH2NH2NaoC,HsAcOHH2CH,NO2一+Ni78~84%
78~84% CH3NO2 + HCHO OH- HOCH2 C NO2 CH2OH CH2OH H2 HOCH2 C NH2 CH2OH CH2OH 3 O + CH3NO2 NaOC2H5 AcOH HO CH2NO2 HO CH2NH2 H2 Ni
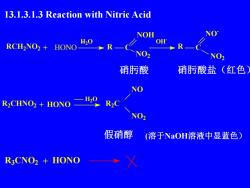
13.1.3.1.3 Reaction with Nitric AcidNONOHH,0OHRCH,NO2+HONONO2NO2硝酸硝酸盐(红色NOH20R2CR,CHNO2 + HONONO2假硝醇(溶于NaOH溶液中显蓝色)R3CNO2 + HONO
13.1.3.1.3 Reaction with Nitric Acid 硝肟酸 硝肟酸盐(红色) 假硝醇 (溶于NaOH溶液中显蓝色) R3CNO2 + HONO RCH2NO2 + HONO H- 2O R C NO2 NOH OH- R C NO - NO2 R2CHNO2 + HONO H2O R2C NO NO2
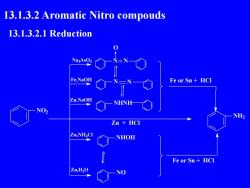
13.1.3.2 Aromatic Nitro compouds13.1.3.2.1ReductionNa3AsO3Fe,NaOHFe or Sn+ HCIZn,NaOHNHNHNO2NH2Zn+ HCIZn,NH.CNHOHFeor Sn+ HCIZn,H,0NO
13.1.3.2 Aromatic Nitro compouds 13.1.3.2.1 Reduction NO2 Na3AsO3 Fe,NaOH Zn,NaOH Zn,NH4Cl Zn,H2O N N O N N NHNH NHOH NO NH2 Fe or Sn + HCl Fe or Sn + HCl Zn + HCl
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十二章 羧酸及其衍生物 Carboxylic Acids and Their Derivatives.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十一章 醛和酮 Aldehydes & Ketones.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第七章 有机波谱分析 Structure Analysis of Organic Compounds by Spectrum.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第五章 脂环烃 Aliphatic Cyclic Hydrocarbons.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第八章 对映异构 Enantiomerism.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第九章 卤代烃 Alkyl Halides.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第二章 烷烃 Alkanes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第四章 炔烃和二烯烃 Alkynes and Dienes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第三章 单烯烃 Alkenes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第一章 绪论 Introduction(主讲:覃兆海).ppt
- 中国农业大学:《有机化学》课程授课教案(讲义)第十八章 周环反应.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十七章 氨基酸、多肽和蛋白质.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十九章 萜类和甾体化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十五章 有机杂环化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十六章 碳水化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十四章 含硫和含磷有机化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十三章 含氮有机化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十二章 羧酸和羧酸衍生物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十章 醇、酚、醚.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十一章 醛和酮.doc
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十章 醇、酚、醚 Alcohols, Phenols and Ethers.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十六章 碳水化合物 Carbohydrates.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十四章 含硫和含磷有机化合物 Sulfur and phophorus organic compounds.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十五章 有机杂环化合物 Heterocyclic Compounds.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十七章 氨基酸、多肽和蛋白质 Amino acids, Peptides and Proteins.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十九章 萜类和甾体化合物 Terpenes and Steroids.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十八章 周环反应 Pericyclic Reactions.ppt
- 《有机化学》课程教学大纲 Organic Chemistry.doc
- 《有机化学》课程授课教案(讲义)第十一章 碳水化合物(糖).doc
- 《有机化学》课程教学资源(文献)课外中英文对照阅读材料.doc
- 《有机化学》课程授课教案(讲义)第四章 对映与非对映异构-精品.doc
- 《有机化学》课程授课教案(讲义)第八章 醛、酮、醌.doc
- 《有机化学》课程授课教案(讲义)第十章 含氮化合物.doc
- 《有机化学》课程授课教案(讲义)第九章 羧酸、羧酸衍生物、取代酸.doc
- 《有机化学》课程授课教案(讲义)第七章 醇、酚、醚.doc
- 《有机化学》课程授课教案(讲义)第六章 卤代烃.doc
- 《有机化学》课程授课教案(讲义)第五章 对映与非对映异构.doc
- 《有机化学》课程授课教案(讲义)第四章 芳香烃.doc
- 《有机化学》课程授课教案(讲义)第一章 绪论.doc
- 《有机化学》课程授课教案(讲义)第二章 烷烃.doc
