中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十一章 醛和酮 Aldehydes & Ketones

Chapter 11Aldehydes&Ketones
Chapter 11 Aldehydes & Ketones
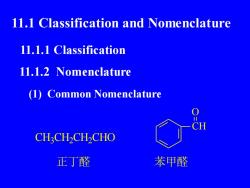
1l.1 Classification and Nomenclature11.1.1 Classification11.1.2 Nomenclature(1) Common NomenclatureICHCH,CH,CH,CHO苯甲醛正丁醛
11.1 Classification and Nomenclature 11.1.1 Classification 11.1.2 Nomenclature (1) Common Nomenclature CH3 CH2 CH2 CHO 正丁醛 CH O 苯甲醛
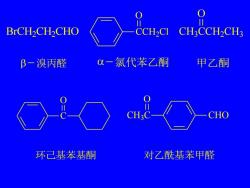
11BrCH,CH,CHOCCH,CICH3CCH2CH3α一氯代苯乙酮甲乙酮β一溴丙醛CH,CCHO对乙酰基苯甲醛环已基苯基酮
BrCH2 CH2 CHO 溴丙醛 CCH2 Cl O 氯代苯乙酮 CH3 CCH2 CH3 O 甲乙酮 C O 环己基苯基酮 CH3 C O CHO 对乙酰基苯甲醛
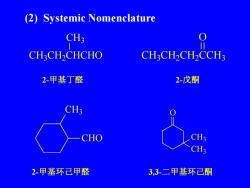
(2) Systemic NomenclatureCH3CH,CH,CHCHOCH,CHCH2CCH32-戊酮2-甲基丁醛CH3CH3CHOCH32-甲基环已甲醛3.3-二甲基环已酮
(2) Systemic Nomenclature CHO CH3 2-甲基环己甲醛 2-甲基丁醛 CH3 CH2 CHCHO CH3 2-戊酮 CH3 CH2 CH2 CCH3 O 3,3-二甲基环己酮 O CH3 CH3

Precedence order of functional groups asprinciplepartin nomenclatureAldehyde>Ketone>Alcohol>AlkeneandAlkyneegCH3CCH,CH2CHCH3LCH,CCH,CH,CHOCH3CH25,6-二甲基-6-庚烯-2-酮4一氧代戊醛
CH3 CCH2 CH2 CHO O 4 氧代戊醛 Aldehyde>Ketone>Alcohol>Alkene and Alkyne Precedence order of functional groups as principle part in nomenclature eg. CH3 CCH2 CH2 CHCH3 O C CH3 CH2 5,6 二甲基 6 庚烯 2 酮
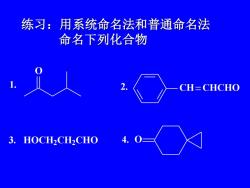
练习:用系统命名法和普通命名法命名下列化合物CH= CHCHOHOCH2CH2CHO3
练习:用系统命名法和普通命名法 命名下列化合物 1. O 2. CH CHCHO 3. HOCH2 CH2 CHO 4. O
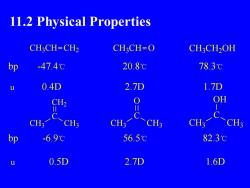
11.2 Physical PropertiesCH3CH=CH2CH3CH=OCH,CH2OH20.8℃78.3℃bp-47.4℃0.4D2.7D1.7DuOHCH2ⅡICCH3CH3CH3CH3CH3CH3-6.9℃82.3℃bp56.5℃0.5D2.7D1.6Du
11.2 Physical Properties CH3CH CH2 CH3CH O CH3CH2OH bp -47.4℃ 20.8℃ 78.3℃ u 0.4D 2.7D 1.7D C O CH3 CH3 C CH2 CH3 CH3 C OH CH3 CH3 bp -6.9℃ 56.5℃ 82.3℃ u 0.5D 2.7D 1.6D

11.3 Chemical PropertiesReactive centers of aldehyde and ketoneQ:← attacked by electrophilic reagent-attackedbynucleophilicreagentR(H)HReactions of aldehydereactions onaα-H
11.3 Chemical Properties Reactive centers of aldehyde and ketone C C H R(H) O - ↑ reactions onα-H ← attacked by electrophilic reagent ←attacked by nucleophilic reagent ↖ Reactions of aldehyde
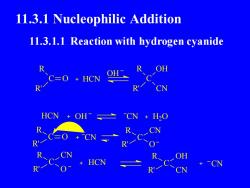
11.3.1 Nucleophilic Addition11.3.1.1 Reaction with hydrogen cyanideOHRROHC=O + HCNRRHCNOHCN+ H20RRCNCNPR0ROHHCN-CN+RRN
11.3.1 Nucleophilic Addition C O R R' HCN C R R' OH CN HCN CN H2 O C R R' O CN OH OH C R R' CN O C R R' CN O HCN C OH R' CN R CN 11.3.1.1 Reaction with hydrogen cyanide
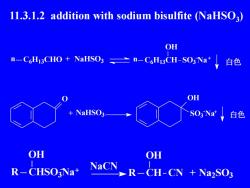
11.3.1.2 addition with sodium bisulfite (NaHSO3)OHn-CHi3CHO + NaHSO3二n-CH3CH-SO3Na白色OH+NaHSO3SO3Nat白色OHOHNaCNR-CHSO:Na+R-CH-CN + Na2SO3
11.3.1.2 addition with sodium bisulfite (NaHSO3 ) n C6H13CHO NaHSO3 n C6H13CH OH + SO3 Na 白色 O NaHSO3 OH SO3 - + Na 白色 CHSO3 Na OH R NaCN CH OH R CN + Na2 SO3
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第七章 有机波谱分析 Structure Analysis of Organic Compounds by Spectrum.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第五章 脂环烃 Aliphatic Cyclic Hydrocarbons.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第八章 对映异构 Enantiomerism.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第九章 卤代烃 Alkyl Halides.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第二章 烷烃 Alkanes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第四章 炔烃和二烯烃 Alkynes and Dienes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第三章 单烯烃 Alkenes.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第一章 绪论 Introduction(主讲:覃兆海).ppt
- 中国农业大学:《有机化学》课程授课教案(讲义)第十八章 周环反应.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十七章 氨基酸、多肽和蛋白质.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十九章 萜类和甾体化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十五章 有机杂环化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十六章 碳水化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十四章 含硫和含磷有机化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十三章 含氮有机化合物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十二章 羧酸和羧酸衍生物.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十章 醇、酚、醚.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第十一章 醛和酮.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第九章 卤代烃.doc
- 中国农业大学:《有机化学》课程授课教案(讲义)第五章 脂环烃.doc
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十二章 羧酸及其衍生物 Carboxylic Acids and Their Derivatives.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十三章 含氮有机化合物 Nitrogen Containing Organic Compounds.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十章 醇、酚、醚 Alcohols, Phenols and Ethers.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十六章 碳水化合物 Carbohydrates.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十四章 含硫和含磷有机化合物 Sulfur and phophorus organic compounds.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十五章 有机杂环化合物 Heterocyclic Compounds.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十七章 氨基酸、多肽和蛋白质 Amino acids, Peptides and Proteins.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十九章 萜类和甾体化合物 Terpenes and Steroids.ppt
- 中国农业大学:《有机化学》课程教学课件(PPT讲稿)第十八章 周环反应 Pericyclic Reactions.ppt
- 《有机化学》课程教学大纲 Organic Chemistry.doc
- 《有机化学》课程授课教案(讲义)第十一章 碳水化合物(糖).doc
- 《有机化学》课程教学资源(文献)课外中英文对照阅读材料.doc
- 《有机化学》课程授课教案(讲义)第四章 对映与非对映异构-精品.doc
- 《有机化学》课程授课教案(讲义)第八章 醛、酮、醌.doc
- 《有机化学》课程授课教案(讲义)第十章 含氮化合物.doc
- 《有机化学》课程授课教案(讲义)第九章 羧酸、羧酸衍生物、取代酸.doc
- 《有机化学》课程授课教案(讲义)第七章 醇、酚、醚.doc
- 《有机化学》课程授课教案(讲义)第六章 卤代烃.doc
- 《有机化学》课程授课教案(讲义)第五章 对映与非对映异构.doc
- 《有机化学》课程授课教案(讲义)第四章 芳香烃.doc
