内蒙古农业大学:《分子生物学》课程教学资源(自编教材)英文版生物化学

内蒙古农业大学 英汉双语教学辅助教材 生物化学选读 冯福应周欢敏主编 内蒙古农业大学教务处 二O0八年三月
内蒙古农业大学 英汉双语教学辅助教材 生物化学选读 冯福应 周欢敏 主编 内蒙古农业大学教务处 二〇〇八年三月

1.Introduction of Biochemistry In biology we are introduced to the extraordinary diversity of living organisms,a diversity that be classified.If we limit our inspection of living things to the gross organismic level,it is easy to be overwhelmed by this complexity.Furthermore,we will not find explanations for why organisms are constructed the way the are at this level of inquiry.If we probe more deeply into organismic structure.we find that cells are composed of a limited number of subcellular structures find the ultimate explanations for organismic structure and behavior.We should revel in the discovery that while living organisms are very complicated,there is a common chemistry and common rules that explain how they work. The object of this text is to acquaint the student of biochemistry with the basic facts and We will begin with an overview of some features of biochemistry as a biological principle. 1.I Biochemistry Is Chemistry of Living Organisms 1.1.1 The Concept of Biochemistry Life phenomena are unique and special motion of matters,in biological terms they are variant century,we now understand life are composed of molecules such as protein,nucleic acid,lipid and etc,and just they work together is to produce rich and colorful metabolism and life.And so,what are these molecules included,how are they generated and disposed and how do they change into and interact between each other?To discover the answers of these questions is the study content of biochemistry. Therefore the biochemistry,aso canbe called biological chemistry,has been defined as the study of the chemical nature of live beings and the chemical rule in the process of life activities. The chemistry of live beings resembles the chemistry of organic reaction at the molecule degree. Though the molecules are lifeless,in appreciate complexity and number they compose the live beings biochemical phenomena with cell biology,physiology,genetics,microbiology and molecular biology.though biochemistry is a basic one. 1.12 Basic chemical properties of living organisms 1.1.2.1 All living organisms consist of variant of biomolecules The diversity of nisms dep asmall bacterium,exampe about five thousands different species of molecules,including three thousands species of protein and one thousand species of nucleic acid.The sizes of biomolecules vary from very small to huge,and they appear in simple type or polymer.All different molecules have their own unique and special function.Interestingly,all
1 1. Introduction of Biochemistry In biology we are introduced to the extraordinary diversity of living organisms, a diversity that is so great that taxonomists generally acknowledge that there are many more species than will ever be classified. If we limit our inspection of living things to the gross organismic level, it is easy to be overwhelmed by this complexity. Furthermore, we will not find explanations for why organisms are constructed the way the are at this level of inquiry. If we probe more deeply into organismic structure, we find that cells are composed of a limited number of subcellular structures, macromolecules and small molecules. We also find that the macromolecules, which make up the bulk of the solid matter of cells, are closely related in structure. It is at the molecular level that we find the ultimate explanations for organismic structure and behavior. We should revel in the discovery that while living organisms are very complicated, there is a common chemistry and common rules that explain how they work. The object of this text is to acquaint the student of biochemistry with the basic facts and principles of this subject. We will begin with an overview of some features of biochemistry as a biological principle. 1.1 Biochemistry Is Chemistry of Living Organisms 1.1.1 The Concept of Biochemistry Life phenomena are unique and special motion of matters; in biological terms they are variant processes of metabolism. Thanks to the investigation of thousand of biochemists over the past century, we now understand life are composed of molecules such as protein, nucleic acid, lipid and, etc, and just they work together is to produce rich and colorful metabolism and life. And so, what are these molecules included, how are they generated and disposed and how do they change into and interact between each other? To discover the answers of these questions is the study content of biochemistry. Therefore the biochemistry, also can be called biological chemistry, has been defined as the study of the chemical nature of live beings and the chemical rule in the process of life activities. The chemistry of live beings resembles the chemistry of organic reaction at the molecule degree. Though the molecules are lifeless, in appreciate complexity and number they compose the live beings. Biochemistry is a growing discipline closely linked to other fields. For this reason, relate the biochemical phenomena with cell biology, physiology, genetics, microbiology and molecular biology, though biochemistry is a basic one. 1.1.2 Basic chemical properties of living organisms 1.1.2.1 All living organisms consist of variant of biomolecules The diversity of organisms depends on molecules diversity and their structural complexity. Take a small bacterium, Escherichia coli, as example, it contains about five thousands different species of molecules, including three thousands species of protein and one thousand species of nucleic acid. The sizes of biomolecules vary from very small to huge, and they appear in simple type or polymer. All different molecules have their own unique and special function. Interestingly, all

organisms practice molecular economy,that they would not construct the complexity over their well-arranged molecu ystems Though large verities of biomolecules exist in organisms,they are well arranged rather than out-of-ordered,even a single-celled organism have well-organized structures.Moreover the biomolecules organization precisely control every biochemical reaction,whatever in a single-celled organisms in which hundreds of reaction undergo at the same time.or in multi-celled ismsown much complicated 1.12.3All living organisms need enzyme as catalyst for metabolic reactior Enzymes function as catalysts that change the rate of a reaction without being change themselves.Virtually all enzymes are proteins.though some catalvtically RNAs have been identified.Enzymes are highly specific and their activity can be regulated.As reaction catalyst,the specialty and efficiency of enzymes far excel those of inorganic catalysts 1.12All living organisms require energy Energy is required to meet bioactivities and the energy conveys are agreed with the Second Law of thermodynamics.All bio-energy is originated from the Sun and the transformation of light energy to chemical energy is carried out by plant and some bacteria with photosynthesis.In food-web,photosynthetic plant and bacteria utilize sun energy to synthesize carbohydrates and act as producer,while animal and other microorganisms eat the above nu s and they play as cons mers 1.1.2.5 All living organisms store their genetic information in genes Self reproducing drive the generation shifts and therefore it is most dominant profile of live beings.The genetic information required by self-reproduce is lied in gene composed of nucleic nces.DNAismain aterials of mjor live beings and t oducing ability As to the features of biochemistry above,biochemistry is divided into three main basic fields chemistry of biomolecule,metabolism(materials and energy)and molecular genetics. 12 The Content and Intention of Biochemistry Biochemistry studies substance composition,propert s.metabolism.molecule structure and function.With the aim the hasic la and studying methods in biology chemis and physics biochemistry study the composition and structure.physical-and-chemical properties structure and function of chemical substance in organisms,from angle of biology and chemistry. respectively. Biochemistry aims to make students known the relationships between/among protein.enzyme nucleic acid,carbohydrate,lipid,vitamin and hormone,gr n of enzyme in catabolism of thr Jor cellula structural substances protein,lipid and carbohydrate,and understood the relationship betweer structure and function of biomacromolecules and basic rule of metabolic regulation.Mainly at the degree of molecule,biochemistry probes the basic structure,space structure:biological properties and function of biomolecu and also at relatively macroscopic cellular and subcellular degree investigate the dist 1.3 The Simple History of Biochemistry As one of most ancient branch of life science,biochemistry was originated from physiology and organic chemistry.Modern biochemistry started at the early time of 18 century and became an
2 organisms practice molecular economy, that they would not construct the complexity over their requirements. 1.1.2.2 All living organisms are complicate and well-arranged molecular systems Though large verities of biomolecules exist in organisms, they are well arranged rather than out-of-ordered, even a single-celled organism have well-organized structures. Moreover the biomolecules organization precisely control every biochemical reaction, whatever in a single-celled organisms in which hundreds of reaction undergo at the same time, or in multi-celled organisms own much complicated organized structures and control systems. 1.1.2.3 All living organisms need enzyme as catalyst for metabolic reaction Enzymes function as catalysts that change the rate of a reaction without being change themselves. Virtually all enzymes are proteins, though some catalytically RNAs have been identified. Enzymes are highly specific and their activity can be regulated. As reaction catalyst, the specialty and efficiency of enzymes far excel those of inorganic catalysts. 1.1.2.4 All living organisms require energy Energy is required to meet bioactivities and the energy conveys are agreed with the Second Law of thermodynamics. All bio-energy is originated from the Sun and the transformation of light energy to chemical energy is carried out by plant and some bacteria with photosynthesis. In food-web, photosynthetic plant and bacteria utilize sun energy to synthesize carbohydrates and act as producer, while animal and other microorganisms eat the above nutrients and they play as consumers. 1.1.2.5 All living organisms store their genetic information in genes Self reproducing drive the generation shifts and therefore it is most dominant profile of live beings. The genetic information required by self-reproduce is lied in gene composed of nucleic sequences. DNA is main genetic materials of major live beings and its self-reproducing ability enables the live beings similar ability. As to the features of biochemistry above, biochemistry is divided into three main basic fields, chemistry of biomolecule, metabolism (materials and energy) and molecular genetics. 1.2 The Content and Intention of Biochemistry Biochemistry studies substance composition, properties, metabolism, molecule structure and function in organisms. With the aim of the basic law and studying methods in biology, chemistry and physics, biochemistry study the composition and structure, physical-and-chemical properties, structure and function of chemical substance in organisms, from angle of biology and chemistry, respectively. Biochemistry aims to make students known the relationships between/among protein, enzyme, nucleic acid, carbohydrate, lipid, vitamin and hormone, grasped the application of enzyme in laboratory and industry, mastered basic rules of anabolism and catabolism of three major cellular structural substances protein, lipid and carbohydrate, and understood the relationship between structure and function of biomacromolecules and basic rule of metabolic regulation. Mainly at the degree of molecule, biochemistry probes the basic structure, space structure; biological properties and function of biomolecule, and also at relatively macroscopic cellular and subcellular degree investigate the distribution and role of biomolecules. 1.3 The Simple History of Biochemistry As one of most ancient branch of life science, biochemistry was originated from physiology and organic chemistry. Modern biochemistry started at the early time of 18 century and became an
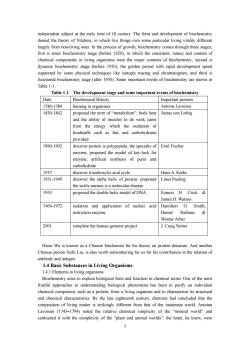
independent subiect at the early time of 18 century.The form and development of biochemistry denied the theory of Vitalims,in which live things ow largely f rom ving ones.In the process of growth, hrough three stage first is static biochemistry stage (before 1920),in which the consistent,nature and content of chemical components in living organisms were the major contents of biochemistry:second is dynamic biochemistry stage (before 1950).the golden period with rapid development speed supported by some physical techniques like isotopic tracing and chromatogram:and third is istry stage (after Some important events of bioche nistry are shown in Table 1-1 Table 1-1 The development stage and some important events of biochemistry Date Biochemical History Important persons 1780.1789 uring in organisms Antoine lavoisier 1830-1842 proposed the term ofmetabolism"body hea Justus von Liebig and the ability of muscles to do work cam from the energy which the oxidation of foodstuffs such as fats and carbohydrate provided 1890-1902 discover protein is polypeptide.the specialtyof Emil Fischer enzyme.proposed the model of key-lock fo enzyme:artificial synthesis of purin and carhohydrate 1037 Hans A.Krebs 1931-1949 discover the alpha helix of protein,proposed Linus Pauling the sickle anemia is a molecular disease 1953 proposed the double helix model of DNA Francis H.Crick& James d watson 1969-1972 isolation and application of nucleic acid Hamilton Smith, restriction enzyme Daniel Nathans Werner Arber 2001 complete the human genome project J.Craig Venter Hsien Wu is known as a Chinese biochemist for his theory on protein denature.And another Chinese person Sizhi Liu,is also worth remembering for us for his contribution in the relation of antibody and antigen 1.4 Basic Substances in Living Organisms 1.4.1 Elements in living organisms Biochemistry aims to explain biological form and function in chemical terms.One of the mos fruitful approaches to understanding biological phenomena has been to purify an individual chemical component.such as a protein.from a living organism and to characterize its structural and chemical characteristics by the late eighteenth century chemists had concluded that the matter is from that of the inanimate world.Antoine Lavoisier (1743-1794)noted the relative chemical simplicity of the "mineral world"and contrasted it with the complexity of the "plant and animal worlds";the latter,he knew,were 3
3 independent subject at the early time of 18 century. The form and development of biochemistry denied the theory of Vitalims, in which live things own some particular living vitality different largely from non-living ones. In the process of growth, biochemistry comes through three stages, first is static biochemistry stage (before 1920), in which the consistent, nature and content of chemical components in living organisms were the major contents of biochemistry; second is dynamic biochemistry stage (before 1950), the golden period with rapid development speed supported by some physical techniques like isotopic tracing and chromatogram; and third is functional biochemistry stage (after 1950). Some important events of biochemistry are shown in Table 1-1. Table 1-1 The development stage and some important events of biochemistry Date Biochemical History Important persons 1780-1789 burning in organisms Antoine Lavoisier 1830-1842 proposed the term of “metabolism”; body heat and the ability of muscles to do work came from the energy which the oxidation of foodstuffs such as fats and carbohydrates provided Justus von Liebig 1890-1902 discover protein is polypeptide, the specialty of enzyme, proposed the model of key-lock for enzyme; artificial synthesis of purin and carbohydrate Emil Fischer 1937 discover tricarboxylic acid cycle Hans A. Krebs 1931-1949 discover the alpha helix of protein; proposed the sickle anemia is a molecular disease Linus Pauling 1953 proposed the double helix model of DNA Francis H. Crick & James D. Watson 1969-1972 isolation and application of nucleic acid restriction enzyme Hamilton O. Smith, Daniel Nathans & Werner Arber 2001 complete the human genome project J. Craig Venter Hsien Wu is known as a Chinese biochemist for his theory on protein denature. And another Chinese person Sizhi Liu, is also worth remembering for us for his contribution in the relation of antibody and antigen. 1.4 Basic Substances in Living Organisms 1.4.1 Elements in living organisms Biochemistry aims to explain biological form and function in chemical terms. One of the most fruitful approaches to understanding biological phenomena has been to purify an individual chemical component, such as a protein, from a living organism and to characterize its structural and chemical characteristics. By the late eighteenth century, chemists had concluded that the composition of living matter is strikingly different from that of the inanimate world. Antoine Lavoisier (1743–1794) noted the relative chemical simplicity of the “mineral world” and contrasted it with the complexity of the “plant and animal worlds”; the latter, he knew, were

composed of compounds rich in the elements carbon.oxygen.nitrogen.and phosphorus.During yeast and n these two apparentl very different cell types,the breakdown of gucose in yeast and invoved the same te chemical intermediates.Subsequent studies of many other biochemical processes in many different organisms have confirmed the generality of this observation,neatly summarized by Jacques Monod:"What is true of E.coli is true of the elephant"The current understanding that all sality of chemical intermediates and transformations.Only abo occurring chemical elements are essential to organisms.Most of the elements in living matter have relatively low atomic numbers:only five have atomic numbers above that of selenium.34 (Fig 1-1).The four most abundant elements in living organisms.in terms of percentage of total number of atoms,are hydrogen,oxygen,nitrogen,and carbon,which together make up more than%of the mass ells They are capable of,and fou bonds,respectively;in general,the lightest elements form the strongest bonds.The trace element (Fig.1-1)represent a miniscule fraction of the weight of the human body,but all are essential to life,usually because they are essential to the function of specific proteins,including enzymes.The oxygen-transporting capacity of the hemoglobin molecule,for example,is absolutely dependent on four iron ions that make up only %fitsmas H 'u‘Re 已race ciement 'B"c 'x "o 'p Al ''s CI Ar k Ca be 'n v Cr Ma Pe Co M Cu in "da Ge be Br kr awzr.tu "it.Ya "Ae "Ca "'In "Sn.B飞 Ce Ba if Ta "W Re Os ir Pe Au ig T Ph Bi Po At in Fig.1-1 Elements essential to animal life and health.Bulk elements (shaded orange)are structural components of cells and tissues and are required in the diet in gram quantities daily.For trace elements (shaded bright yellow),the requirer nis are much er:for hu ans,a few milligrams per day of F Cu,and Zn,even less of the others.The elemental requirements for plants and microorganisms are similar to those shown here;the ways in which they acquire these elements vary. 1.4.2 Substances in living organisms 1.4.2.1Water
4 composed of compounds rich in the elements carbon, oxygen, nitrogen, and phosphorus. During the first half of the twentieth century, parallel biochemical investigations of glucose breakdown in yeast and in animal muscle cells revealed remarkable chemical similarities in these two apparently very different cell types; the breakdown of glucose in yeast and muscle cells involved the same ten chemical intermediates. Subsequent studies of many other biochemical processes in many different organisms have confirmed the generality of this observation, neatly summarized by Jacques Monod: “What is true of E. coli is true of the elephant.” The current understanding that all organisms share a common evolutionary origin is based in part on this observed universality of chemical intermediates and transformations. Only about 30 of the more than 90 naturally occurring chemical elements are essential to organisms. Most of the elements in living matter have relatively low atomic numbers; only five have atomic numbers above that of selenium, 34 (Fig. 1–1). The four most abundant elements in living organisms, in terms of percentage of total number of atoms, are hydrogen, oxygen, nitrogen, and carbon, which together make up more than 99% of the mass of most cells. They are the lightest elements capable of forming one, two, three, and four bonds, respectively; in general, the lightest elements form the strongest bonds. The trace elements (Fig. 1–1) represent a miniscule fraction of the weight of the human body, but all are essential to life, usually because they are essential to the function of specific proteins, including enzymes. The oxygen-transporting capacity of the hemoglobin molecule, for example, is absolutely dependent on four iron ions that make up only 0.3% of its mass. Fig. 1–1 Elements essential to animal life and health. Bulk elements (shaded orange) are structural components of cells and tissues and are required in the diet in gram quantities daily. For trace elements (shaded bright yellow), the requirements are much smaller: for humans, a few milligrams per day of Fe, Cu, and Zn, even less of the others. The elemental requirements for plants and microorganisms are similar to those shown here; the ways in which they acquire these elements vary. 1.4.2 Substances in living organisms 1.4.2.1 Water

No doubt,no water no life.Water is most abundant substance in living organisms,making up %or more 学nD in aqueous medium in which life began Water distribute in all parts of cell.as a mediate of metabolic reaction.energy transmission and substances transportation.Major biochemical reactions of organisms operate in water systems.and in many reactions water is not only reactor but also product.In biological reaction water balances the pH with acidic and basic sub stances to maintain a special pH for cell to allow for the r eact system having buffer capacity.Water is also as a major structural component of organs or tissues All the function owned by water is due to its polarity and cohesion. 1.4.2.2 Inorganic compounds and salts Metal ions.organic salts.enzyme activators complexes and etc are maior forms of inorganic eompounds and salts in living cell,with reulate the osmotic balance outside and inside and assist nutrient transportation.Analysis of cell inorganic salts can show the nutrient state of the cell and then can be used as determining evidence for element supply,esp.in agricultural plant production. 1.4.2.3 Micromolecular organic compounds Dissolved in theaqueous phase (cytosol)of all cells of small organic molecules(M 100 to 50)the in the major nearly every cel the metabolites and pathways that have been conserved throughout the course of evolution.(See Box 1-1 for an explanation of the various ways of referring to molecular weight This collection of molecules includes the common amino acids,nucleotides,sugars and their phosphorylated derivatives,and a number of,and tricarboxylic acids.The polar or charged. present in micromolar concentrations.They are trapped within the cell because the plasma membrane is impermeable to them-although specific membrane transporters can catalyze the movement of some molecules into and out of the cell or between compartments in eukarvotic cells.The universal occurrence of the same set of compounds in living cells is a manifestation of the universality of metabolic design vation of metabolic pathways that developed in the earliest cell There ar eother small tocertain types of celsor For exampl vascular plants contain,in addition to the universal set,small molecules called secondary metabolites.which play a role specific to plant life.These metabolites include compounds that give plants their characteristic scents,and compounds such as morphine,quinine,nicotine,and caffeine that are valuedfor their physiological humans but used for other purposes by plants.The entire olle olecules in a given cell as been called metabolome,in parallel with the term "genome".If we knew the composition of a cell's metabolome.we could predict which enzymes and metabolic pathways were active in that cell 1.4.2.4 Macromolecular organic compounds As we have known from the above,the major precursors for the formation of biomolecules are monium (NH).nitrate(NO and dinitrogen(N)which areall simple molecules and most of them needed tobe resembe to form macromolecular organic compounds.Before the conversions,metabolic processes assimilate and transform these inorganic precursors through ever more complex levels of biomolecular order.In the first step,precursors are converted to metabolites,simple organic 5
5 No doubt, no water no life. Water is most abundant substance in living organisms, making up 70% or more in most of organisms. The first living organisms doubtless arose in an aqueous environment, and the course of evolution has been shaped by the properties of the aqueous medium in which life began. Water distribute in all parts of cell, as a mediate of metabolic reaction, energy transmission and substances transportation. Major biochemical reactions of organisms operate in water systems, and in many reactions water is not only reactor but also product. In biological reaction water balances the pH with acidic and basic substances to maintain a special pH for cell to allow for the reaction system having buffer capacity. Water is also as a major structural component of organs or tissues. All the function owned by water is due to its polarity and cohesion. 1.4.2.2 Inorganic compounds and salts Metal ions, organic salts, enzyme activators, complexes, and etc. are major forms of inorganic compounds and salts in living cell, with those to operate their physiological function. Inorganic slats also can regulate the osmotic balance outside and inside of cell and assist nutrients transportation. Analysis of cell inorganic salts can show the nutrient state of the cell and then can be used as determining evidence for element supply, esp. in agricultural plant production. 1.4.2.3 Micromolecular organic compounds Dissolved in the aqueous phase (cytosol) of all cells is a collection of 100 to 200 different small organic molecules (Mr ~100 to ~500), the central metabolites in the major pathways occurring in nearly every cell—the metabolites and pathways that have been conserved throughout the course of evolution. (See Box 1–1 for an explanation of the various ways of referring to molecular weight.) This collection of molecules includes the common amino acids, nucleotides, sugars and their phosphorylated derivatives, and a number of mono-, di-, and tricarboxylic acids. The molecules are polar or charged, water soluble, and present in micromolar to millimolar concentrations. They are trapped within the cell because the plasma membrane is impermeable to them—although specific membrane transporters can catalyze the movement of some molecules into and out of the cell or between compartments in eukaryotic cells. The universal occurrence of the same set of compounds in living cells is a manifestation of the universality of metabolic design, reflecting the evolutionary conservation of metabolic pathways that developed in the earliest cells. There are other small biomolecules, specific to certain types of cells or organisms. For example, vascular plants contain, in addition to the universal set, small molecules called secondary metabolites, which play a role specific to plant life. These metabolites include compounds that give plants their characteristic scents, and compounds such as morphine, quinine, nicotine, and caffeine that are valued for their physiological effects on humans but used for other purposes by plants. The entire collection of small molecules in a given cell has been called that cell’s metabolome, in parallel with the term “genome”. If we knew the composition of a cell’s metabolome, we could predict which enzymes and metabolic pathways were active in that cell. 1.4.2.4 Macromolecular organic compounds As we have known from the above, the major precursors for the formation of biomolecules are water, carbon dioxide, and three inorganic nitrogen compounds—ammonium (NH4 +), nitrate(NO3 - ), and dinitrogen (N2), which are all simple molecules and most of them needed to be resembled to form macromolecular organic compounds. Before the conversions, metabolic processes assimilate and transform these inorganic precursors through ever more complex levels of biomolecular order. In the first step, precursors are converted to metabolites, simple organic

compounds that are intermediates in cellular energy transformation and in the biosynthesis of various sets of building blocks amino acids,sugars,nucleotides,fatty acids,and glycerol.By covalent linkage of these blocks.th re constructed:proteins polysaccharides,polynucleotides (DNA and RNA),and lipids Interactions among macromolecules lead to the next level of structural organization,supramolecular complexes.Here, various members of one or more of the classes of macromolecules come together to form specific assemblies serving important subcellular functions.Examples of these supramolecular assemblies aremultifunction zyme complexes,ribosomes,chro es,and cytoskeletal elements.For exampe,a ukaryotic ibsome four RNA molecules and at least7 uniqu proteins.These supramolecular assemblies are an interesting contrast to their components because their structural integrity is maintained by noncovalent forces,not by covalent bonds.These noncovalent forces include hydrogen bonds.ionic attractions.Van der Waals forces.and hydrophobic interactions between macromolecules.Such forces maintain these supramolecular ovaent forces are weak (essth architecture of the supramolecular complex under conditions of temperature,pH,and ionic strength that are consistent with cell life. Review Questions 1.List five principles that are central to our current understanding of living organisms. 2.List your suggestions for the course of biochemistry. References 1.X.R.Wu,Essential Biochemistry,China Agriculture Publishing Company,1999 2.T.MeKee J.R.MeKee,Biochemistry:an introduction,second edition,MeGraw-Hill companies,Inc,2001. 6
6 compounds that are intermediates in cellular energy transformation and in the biosynthesis of various sets of building blocks: amino acids, sugars, nucleotides, fatty acids, and glycerol. By covalent linkage of these building blocks, the macromolecules are constructed: proteins, polysaccharides, polynucleotides (DNA and RNA), and lipids. Interactions among macromolecules lead to the next level of structural organization, supramolecular complexes. Here, various members of one or more of the classes of macromolecules come together to form specific assemblies serving important subcellular functions. Examples of these supramolecular assemblies are multifunctional enzyme complexes, ribosomes, chromosomes, and cytoskeletal elements. For example, a eukaryotic ribosome contains four different RNA molecules and at least 70 unique proteins. These supramolecular assemblies are an interesting contrast to their components because their structural integrity is maintained by noncovalent forces, not by covalent bonds. These noncovalent forces include hydrogen bonds, ionic attractions, Van der Waals forces, and hydrophobic interactions between macromolecules. Such forces maintain these supramolecular assemblies in a highly ordered functional state. Although noncovalent forces are weak (less than 40 kJ/mol), they are numerous in these assemblies and thus can collectively maintain the essential architecture of the supramolecular complex under conditions of temperature, pH, and ionic strength that are consistent with cell life. Review Questions 1. List five principles that are central to our current understanding of living organisms. 2. List your suggestions for the course of biochemistry. References 1. X. R. Wu, Essential Biochemistry, China Agriculture Publishing Company, 1999. 2. T. McKee & J. R. McKee, Biochemistry: an introduction, second edition, McGraw-Hill companies, Inc, 2001

2.Proteins Proteins are a diverse and abundant class of biomolecules,constituting more than 50%of the dryweigh of cls.This diversity and of proteins aspects of cell structure and function.An extraordinary diversity of cellular activity is possibl only because of the versatility inherent in proteins,each of which is specifically tailored to its biological role.The pattern by which each is tailored resides within the genetic information of cells,encoded in a specific sequence of nucleotide bases in DNA.Each such segment of encoded ression of the gene leads to synthesis of th enoded by the cell with the functions to that particular protein Proteins are the agents of biological function;they are also the expressions of genetic information. 2.1 Introduetion to proteins Almost everything that occurs in the cell involves one or more proteins.Proteins provide ,cataye celar reactions,and myriad of other tasks.Their cen place in protein there is a segment of DNA (a gene;see Chapter 4)that encodes information specifying its sequence of amino acids.There are thousands of different kinds of proteins in a typical cell each encoded by a gene and each performing a specific function.Proteins are among the most abundant biological macromolecules and are also extremely versatile in their functions 2.11.1 Elemental composition of proteins Many proteins have been characterized with their purified crystal state.Different from saccharides and lipids in element composition,proteins have nitrogen and some small amount of sulfur beside carbon,hydrogen and oxygen,based on elemental analysis.Some other elements alsoare found in proteins,phosphor iron,copper,iodine,zinc,molybdenum,and er Major Carbon:50%-55% Hydrogen:6%-8% 0ygen:20%-23% Sulfur:0%-4% Nitrogen:16%-18% Average content of nitrogen 16%means that 1g nitrogen equals to 6.25g protein,which is the basis of Kjedahl nitrogen-determining of quantifying proteins. 2.1.1.2 Products and molecules of degrading proteins Primarily degradation of proteins will produce amino acids.that means that these molecules are the basic comp nents of proteins.There are ony acids in different resoures of proteins Except of in proteins acids That isa hydrogen of carbonyl acid been substituted by an amino group. 2.1.2 Types of proteins and their functions We can classify proteins according to their biological roles
1 2. Proteins Proteins are a diverse and abundant class of biomolecules, constituting more than 50% of the dry weight of cells. This diversity and abundance reflect the central role of proteins in virtually all aspects of cell structure and function. An extraordinary diversity of cellular activity is possible only because of the versatility inherent in proteins, each of which is specifically tailored to its biological role. The pattern by which each is tailored resides within the genetic information of cells, encoded in a specific sequence of nucleotide bases in DNA. Each such segment of encoded information defines a gene, and expression of the gene leads to synthesis of the specific protein encoded by it, endowing the cell with the functions unique to that particular protein. Proteins are the agents of biological function; they are also the expressions of genetic information. 2.1 Introduction to proteins Almost everything that occurs in the cell involves one or more proteins. Proteins provide structure, catalyze cellular reactions, and carry out a myriad of other tasks. Their central place in the cell is reflected in the fact that genetic information is ultimately expressed as protein. For each protein there is a segment of DNA (a gene; see Chapter 4) that encodes information specifying its sequence of amino acids. There are thousands of different kinds of proteins in a typical cell, each encoded by a gene and each performing a specific function. Proteins are among the most abundant biological macromolecules and are also extremely versatile in their functions. 2.1.1 Chemical composition and types of Proteins 2.1.1.1 Elemental composition of proteins Many proteins have been characterized with their purified crystal state. Different from saccharides and lipids in element composition, proteins have nitrogen and some small amount of sulfur beside carbon, hydrogen and oxygen, based on elemental analysis. Some other elements also are found in proteins, e.g. phosphor, iron, copper, iodine, zinc, molybdenum, and etc. Major elements in proteins show their ratios as followed, Carbon: 50%-55% Hydrogen: 6%-8% Oxygen: 20%-23% Sulfur: 0%-4% Nitrogen: 16%-18% Average content of nitrogen 16% means that 1g nitrogen equals to 6.25g protein, which is the basis of Kjedahl nitrogen-determining of quantifying proteins. 2.1.1.2 Products and molecules of degrading proteins Primarily degradation of proteins will produce amino acids, that means that these molecules are the basic components of proteins. There are only 20 Amino acids in different resources of proteins. Except of proline, all amino acids in proteins are α-amino acids. That is a hydrogen in α carbon of carbonyl acid been substituted by an amino group. 2.1.2 Types of proteins and their functions We can classify proteins according to their biological roles

Enzymes The most varied and most highly specialized proteins are those with catalytic activity-the enzymes.Virtually all the chemical reactions of organic biomolecules in cells are eatalyzed by enzyme Many the ands of different enzymes,each capableo catalyzing a Transport Proteins Transport proteins in blood plasma bind and carry specific molecules or ions from one organ to another.Hemoglobin of erythrocytes(Fig.2-lb)binds oxygen as the blood passes through the lungs.carries it to the peripheral tissues.and there releases it to participate in theenergy-yiedingxition of rient.Theblod plasma contains lipoproteins,which carry lipids from the organs.Other kinds of transport proteins are present in the plasm membranes and intracellular membranes of all organisms;these are adapted to bind glucose. amino acids.or other substances and transport them across the membrane. Nutrient and Storage Proteins The seeds of many plants store nutrient proteins required for the owth of the germinating seeding Particularly well-studied examples are theseedo min themajor protein the major protei milk,are other examples of nutrient proteins (Fig.2-lc).The ferritin found in some bacteria and ir plant and animal tissues stores iron. Contractile or Motile Proteins Some proteins endow cells and organisms with the ability to ochangto move abo Actin andyhe onractle te of skeletal muscle and also in man nonmuscle protein from which microtubulesare but Microtubules act the protein dynein in ato propel cells. Structural Proteins Many proteins serve as supporting filaments,cables,or sheets,to give biological structures strength or protection.The major component of tendons and cartilage is the fibrous protein which has very high tensile streng th.Leather is amost pure collager Ligaments contair lastin,a structural protein capable of stretching in two dimensions.Hai fingernails,and feathers consist largely of the tough,insoluble protein keratin.The major component of silk fibers and spider webs is fibroin.The wing hinges of some insects are made of resilin,which has nearly perfect elastic properties. Defense Proteins Many proteins defend organisms against invasion by other species or protect them from injury The or antiboc specialized protei nade by the lymphocytes of vertebrates,can recognize and precipitate or neutralize invading bacteria,viruses or foreign proteins from another species.Fibrinogen and thrombin are blood-clotting proteins that prevent loss of blood when the vascular system is injured.Snake venoms,bacterial toxins,and toxic plant proteins.such as ricin,also appear to have defensive functions.Some of these. gen,thrombin,and som noms,are also en ymes Proteins Some proteins help regulate cellular or physiological activity.Among them are many hormones.Examples include insulin,which regulates sugar metabolism,and the growth hormone of the pituitary.The cellular response to many hormonal signals is often mediated by a class of GTP-binding proteins called G proteins (GTP is closely related to ATP. with guanine replacing the adenine portion of the mol proteins bind to DNA and reuate the biosynthesis of enymes and RNA molecule involved in division in both prokaryotes and eukaryotes. Other Proteins There are numerous other proteins whose functions are rather exotic and not easily classified.Monellin,a protein of an African plant,has an intensely sweet taste.It is being 2
2 Enzymes The most varied and most highly specialized proteins are those with catalytic activity-the enzymes. Virtually all the chemical reactions of organic biomolecules in cells are catalyzed by enzymes. Many thousands of different enzymes, each capable of catalyzing a different kind of chemical reaction, have been discovered in different organisms (Fig. 2-la). Transport Proteins Transport proteins in blood plasma bind and carry specific molecules or ions from one organ to another. Hemoglobin of erythrocytes (Fig. 2-lb) binds oxygen as the blood passes through the lungs, carries it to the peripheral tissues, and there releases it to participate in the energy-yielding oxidation of nutrients. The blood plasma contains lipoproteins, which carry lipids from the liver to other organs. Other kinds of transport proteins are present in the plasma membranes and intracellular membranes of all organisms; these are adapted to bind glucose, amino acids, or other substances and transport them across the membrane. Nutrient and Storage Proteins The seeds of many plants store nutrient proteins required for the growth of the germinating seedling. Particularly well-studied examples are the seed proteins of wheat, corn, and rice. Ovalbumin, the major protein of egg white, and casein, the major protein of milk, are other examples of nutrient proteins (Fig. 2-lc). The ferritin found in some bacteria and in plant and animal tissues stores iron. Contractile or Motile Proteins Some proteins endow cells and organisms with the ability to contract, to change shape, or to move about. Actin and myosin function in the contractile system of skeletal muscle and also in many nonmuscle cells. Tubulin is the protein from which microtubules are built. Microtubules act in concert with the protein dynein in flagella and cilia to propel cells. Structural Proteins Many proteins serve as supporting filaments, cables, or sheets, to give biological structures strength or protection. The major component of tendons and cartilage is the fibrous protein collagen, which has very high tensile strength. Leather is almost pure collagen. Ligaments contain elastin, a structural protein capable of stretching in two dimensions. Hair, fingernails, and feathers consist largely of the tough, insoluble protein keratin. The major component of silk fibers and spider webs is fibroin. The wing hinges of some insects are made of resilin, which has nearly perfect elastic properties. Defense Proteins Many proteins defend organisms against invasion by other species or protect them from injury. The immunoglobulins or antibodies, specialized proteins made by the lymphocytes of vertebrates, can recognize and precipitate or neutralize invading bacteria, viruses, or foreign proteins from another species. Fibrinogen and thrombin are blood-clotting proteins that prevent loss of blood when the vascular system is injured. Snake venoms, bacterial toxins, and toxic plant proteins, such as ricin, also appear to have defensive functions. Some of these, including fibrinogen, thrombin, and some venoms, are also enzymes. Regulatory Proteins Some proteins help regulate cellular or physiological activity. Among them are many hormones. Examples include insulin, which regulates sugar metabolism, and the growth hormone of the pituitary. The cellular response to many hormonal signals is often mediated by a class of GTP-binding proteins called G proteins (GTP is closely related to ATP, with guanine replacing the adenine portion of the molecule; Other regulatory proteins bind to DNA and regulate the biosynthesis of enzymes and RNA molecules involved in cell division in both prokaryotes and eukaryotes. Other Proteins There are numerous other proteins whose functions are rather exotic and not easily classified. Monellin, a protein of an African plant, has an intensely sweet taste. It is being

studied as a nonfattening.nontoxic food sweetener for human use.The blood plasma of some Antarctic fish contains antifreeze proteins which protect their blood from freezing Itis extraordinary that all these proteins,with their very different properties and functions,are ma from the same group of 20 amino acids Fig.2-1 Some functions of proteins.(a)The light produced by fireflies is th result of a reaction involving the protein luciferin and ATP,catalyzed by the enzyme luciferase (see Box 13-2).(b)Erythrocytes contain large amounts of the oxygen-transporting protein hemoglobin.(c)The protein keratin.formed by all vertebrates,is the chief structural component of hair,sales,orn,wool ails,and feath s.The black rhinoceros is nearing extinetion in the because of the belief prevalent in some parts of the world that a powder derived from its horn has aphrodisiac properties.In reality,the chemical properties of powdered rhinoceros horn are no different from thoseof vdered bovine hooves or human fingernails. 2.2 Amino acid Proteins are the indispensable agents of biological function,and amino acids are the building blocks of proteins.Proteins are polymers of amino acids.with each amino acid residue joined to its neighbor by a specific type of covalent bond.(The term"residue"reflects the loss of the amino acid is joined to another.)The stunning diversity of the found in nature arises from the intrinsic properties of nly 20 commonl occurring amino acids.These features include(1)the capacity to polymerize,(2)novel acid-base properties,(3)varied structure and chemical functionality in the amino acid side chains,and (4) chirality. 2.2.1 Hydrolysis of proteins Peptide bonds of proteinsare hydrolyzed by either strongacid or strong base.Because acid hydrolysis proceeds without racemization and with less destrction of certain amino acids(Se Thr.Arg.and Cys)than alkaline treatment.it is the method of choice in analysis of the amino acid composition of proteins and polypeptides.Typically,samples of a protein are hydrolyzed with 6N HCI at 110C for 24.48.and 72 hr in sealed glass vials.Tryptophan is destroved by acid and must total amin mposition.The H-oniningnnnrre swy destroyed,but the the three time points(24,48,and 72 hr)allow extrapolation to zero time to estimate the original Ser and Thr content(Fig.2-2).In contrast,peptide bonds involving hydrophobic residues such as valine and isoleucine are only slowly hydrolyzed in acid.Another complication arises because the
3 studied as a nonfattening, nontoxic food sweetener for human use. The blood plasma of some Antarctic fish contains antifreeze proteins, which protect their blood from freezing. It is extraordinary that all these proteins, with their very different properties and functions, are mad from the same group of 20 amino acids. a b c Fig. 2–1 Some functions of proteins. (a) The light produced by fireflies is the result of a reaction involving the protein luciferin and ATP, catalyzed by the enzyme luciferase (see Box 13–2). (b) Erythrocytes contain large amounts of the oxygen-transporting protein hemoglobin. (c) The protein keratin, formed by all vertebrates, is the chief structural component of hair, scales, horn, wool, nails, and feathers. The black rhinoceros is nearing extinction in the wild because of the belief prevalent in some parts of the world that a powder derived from its horn has aphrodisiac properties. In reality, the chemical properties of powdered rhinoceros horn are no different from those of powdered bovine hooves or human fingernails. 2.2 Amino acids Proteins are the indispensable agents of biological function, and amino acids are the building blocks of proteins. Proteins are polymers of amino acids, with each amino acid residue joined to its neighbor by a specific type of covalent bond. (The term “residue” reflects the loss of the elements of water when one amino acid is joined to another.)The stunning diversity of the thousands of proteins found in nature arises from the intrinsic properties of only 20 commonly occurring amino acids. These features include (1) the capacity to polymerize, (2) novel acid–base properties, (3) varied structure and chemical functionality in the amino acid side chains, and (4) chirality. 2.2.1 Hydrolysis of proteins Peptide bonds of proteins are hydrolyzed by either strong acid or strong base. Because acid hydrolysis proceeds without racemization and with less destruction of certain amino acids (Ser, Thr, Arg, and Cys) than alkaline treatment, it is the method of choice in analysis of the amino acid composition of proteins and polypeptides. Typically, samples of a protein are hydrolyzed with 6 N HCl at 110°C for 24, 48, and 72 hr in sealed glass vials. Tryptophan is destroyed by acid and must be estimated by other means to determine its contribution to the total amino acid composition. The OH-containing amino acids serine and threonine are slowly destroyed, but the data obtained for the three time points (24, 48, and 72 hr) allow extrapolation to zero time to estimate the original Ser and Thr content (Fig. 2-2). In contrast, peptide bonds involving hydrophobic residues such as valine and isoleucine are only slowly hydrolyzed in acid. Another complication arises because the
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 内蒙古农业大学:《分子生物学》课程教学实验指导(共六个实验).pdf
- 内蒙古农业大学:《分子生物学》课程教学资源(授课教案).pdf
- 内蒙古农业大学:《分子生物学》课程教学大纲(负责人:李国婧).pdf
- 安徽大学:《基因工程》课程授课教案(讲义,共八章).docx
- 安徽大学:《基因工程》课程教学大纲.pdf
- 安徽大学:《发酵工程》课程各章思考题.doc
- 安徽大学:《发酵工程》课程教学课件(PPT讲稿)发酵培养基的灭菌及相关设备.ppt
- 安徽大学:《发酵工程》课程教学大纲.doc
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)RNA干扰(RNA interference,RNAi).ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第十三章 表面展示技术(外源基因的表面展示 The Surface display of foreign gene).ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第十二章 蛋白质组学.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第十一章 基因诊断与基因治疗.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第十章 转基因动物技术.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第九章 基因工程与干细胞工程.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第八章 基因芯片.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第七章 核酸的分子杂交.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第六章 基因文库构建与筛选.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第五章 核酸的序列测定.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第四章 核酸的体外扩增.ppt
- 重庆医科大学:《分子生物学》课程教学课件(PPT讲稿)第三章 基因表达的调控.ppt
- 内蒙古农业大学:《分子生物学》课程教学资源(自编教材)英文版分子生物学.pdf
- 内蒙古农业大学:《分子生物学》课程教学资源(自编教材)分子生物学实验技术指导.pdf
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 4 DNA Damage and Repair.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 2 Structure of Genome.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 1 Introduction of Molecular Biology.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 3 DNA replication.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 6 The Biosynthesis of Protein(Translation).ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 5 Biosynthesis of RNA.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 9 Principles and Techniques of Gene Engineering.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 7 The Control of Gene Expression.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 8 The Hot Topics in Genomics and Post-Genome Era.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 4 DNA damage and repair.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 3 DNA replication.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 1 Introduction of Molecular Biology.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 2 Structure of Genome.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chaptor 6 The biosynthesis of protein(translation).ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 5 Biosynthesis of RNA.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 8 the hot topics in genomics and Post-Genome Era.ppt
- 《分子生物学》课程教学课件(PPT讲稿)Chapter 7 the control of gene expression.ppt
- 内蒙古科技大学:《微生物学》课程教学大纲 Microbiology A(负责人:马利兵).doc
