《微生物学 Microbiology》课程PPT教学课件(英文版)chemical principles 2/2

Chapter 2,part B Chemical Principles
Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings B.E Pruitt & Jane J. Stein Chapter 2, part B Chemical Principles
Important Biological Molecules Organic compounds always contain carbon and hydrogen. Inorganic compounds typically lack carbon
Important Biological Molecules • Organic compounds always contain carbon and hydrogen. • Inorganic compounds typically lack carbon

Inorganic Compounds:Water 。Polar molecule H (a) Figure 2.4a
• Polar molecule Inorganic Compounds: Water Figure 2.4a
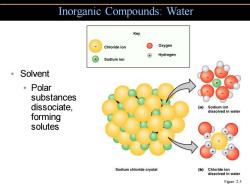
Inorganic Compounds:Water Key Chloride ion g Oxygen ⊕ Hydrogen Sodium ion 。Solvent 。Polar substances dissociate, (a)Sodium ion dissolved in water forming solutes Sodium chloride crystal (b)Chloride ion dissolved in water Figure 2.5
• Solvent • Polar substances dissociate, forming solutes Inorganic Compounds: Water Figure 2.5

Inorganic Compounds:Water H+and OH-participate in chemical reactions
• H+ and OH− participate in chemical reactions Inorganic Compounds: Water
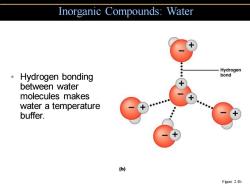
Inorganic Compounds:Water -Hydrogen ·Hydrogen bonding bond between water molecules makes water a temperature buffer. (b) Figure 2.4b
• Hydrogen bonding between water molecules makes water a temperature buffer. Inorganic Compounds: Water Figure 2.4b

Acids,Bases,and Salts HCI 。An acid is a substance that dissociates into one or more H+. HCI→H++CH H+ (a】Acid Figure 2.6a
• An acid is a substance that dissociates into one or more H+. HCl → H+ + Cl− Acids, Bases, and Salts Figure 2.6a
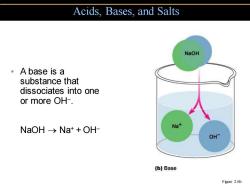
Acids.Bases,and Salts NaOH 。A base is a substance that dissociates into one or more OH-. NaOH->Na++OH- Na+ OH- (b)Base Figure 2.6b
• A base is a substance that dissociates into one or more OH− . NaOH → Na+ + OH− Acids, Bases, and Salts Figure 2.6b

Acids,Bases,and Salts NaCI 。A salt is a substance that dissociates into cations and anions, neither of which is H+ or OH-. Na+ NaCl→Nat+CH (c)Salt Figure 2.6c
• A salt is a substance that dissociates into cations and anions, neither of which is H+ or OH− . NaCl → Na+ + Cl− Acids, Bases, and Salts Figure 2.6c

Acid-Base Balance The amount of H+in a solution is expressed as pH. 。pH=-log[Ht] Increasing [H+],increases acidity. Increasing [OH-]increases alkalinity. Most organisms grow best between pH 6.5 and 8.5
• The amount of H+ in a solution is expressed as pH. • pH = −log[H+] • Increasing [H+], increases acidity. • Increasing [OH− ] increases alkalinity. • Most organisms grow best between pH 6.5 and 8.5. Acid-Base Balance
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《微生物学 Microbiology》课程PPT教学课件(英文版)chemical principles 1/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)biotechnology and recombinant DNA 2/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)biotechnology and recombinant DNA 1/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)applied and industrial microbiology.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)antimicrobial drugs 2/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)antimicrobial drugs 1/2.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第四章 微生物的生长和环境条件.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第六章 真核微生物.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第八章 微生物的生态.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第五章 原核生物.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第三章 细菌的遗传和变异.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第七章 病毒.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)绪论(主讲:刘雅婷).ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第二章 微生物的营养和代谢.ppt
- 云南农业大学:《微生物学》课程教学资源(PPT课件)第一章 微生物的细胞.ppt
- 《微生物学》课程教学资源(PPT课件)第八章 微生物生态.ppt
- 《微生物学》课程教学资源(PPT课件)第九章 传染与免疫.ppt
- 《微生物学》课程教学资源(PPT课件)第七章 微生物的遗传变异和育种.ppt
- 《微生物学》课程教学资源(PPT课件)第四、五章 微生物的营养与培养基.ppt
- 《微生物学》课程教学资源(PPT课件)第六章 微生物的生长繁殖及其控制.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)classification of microrganisms 1/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)classification of microrganisms 2/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)disorders associated with the immune system 1/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)disorders associated with the immune system 2/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)domains bacteria archaea 1/5.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)domains bacteria archaea 2/5.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)domains bacteria archaea 3/5.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)domains bacteria archaea 4/5.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)domains bacteria archaea 5/5.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)environmental microbiology 1/3.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)environmental microbiology 2/3.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)environmental microbiology 3/3.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)functional anatomy of prokaryotic and eukaryotic cells 1/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)functional anatomy of prokaryotic and eukaryotic cells 2/2.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)fungi algae protozoa and helminths 1/4.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)fungi algae protozoa and helminths 2/4.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)fungi algae protozoa and helminths 3/4.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)fungi algae protozoa and helminths 4/4.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)microbial diseases of digestive system 1/3.ppt
- 《微生物学 Microbiology》课程PPT教学课件(英文版)microbial diseases of digestive system 2/3.ppt
