上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.4-6.9)

Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter 6.5 Quantum Mechanical Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations Periodic Table Chapter 6
Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter Chapter 6 6.5 Quantum Mechanical & Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations & Periodic Table
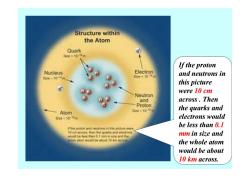
Structure within the Atom Quark 8a0<10增m If the proton Nucleus Electron and neutrons in 6m50-14% 2e《40得mt this picture were 10 cm Neutron and across.Then Proton 520.1013a the quarks and Atom 5w-100m electrons would be less than 0.I 算e proton and0ebgn裤的is picture were 10 cm across.men the quarks and electrone woold bo les5an01m两n size and me mm in size and y#om wou创be心0410mcD the whole atom would be about 10 km across
If the proton and neutrons in this picture were 10 cm across . Then the quarks and electrons would be less than 0.1 mm in size and the whole atom would be about 10 km across
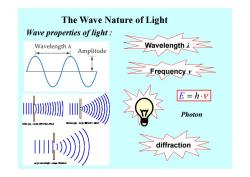
The Wave Nature of Light Wave properties of light Wavelength A Amplitude Wavelongh L E=h.v Photon Wide gop-small efec u》
The Wave Nature of Light Wave properties of light : Wavelength λ Frequency ν diffraction E = ⋅ h ν Photon
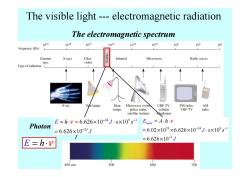
The visible light---electromagnetic radiation The electromagnetic spectrum 1020 1018 1016 1014 1012 1010 103 106 104 Frequency (Hz) Gamma X rays Ultra- alq!s!A Infrared Microwave Radio waves rays violet Type of radiation L X ray Sun lamps Heat Microwave ovens UHF TV. FM radio, AM lamps police radar. cellular VHF TV radio satellite stations tolephones E=hy=6.626×10-34J.s×109s1 Emole =A.h.v Photon =6.626×10-25J =6.02×1023×6.626×10-34J·s×10°s1 E=h.v =6.626×10-2J 400nm 500 600 700
The electromagnetic spectrum The visible light --- electromagnetic radiation E = ⋅ h ν Photon 34 9 1 25 6.626 10 10 6.626 10 E J h s s J ν − − − = ⋅ × ⋅ × = × = 23 34 9 1 2 6.02 10 6.626 10 10 6.626 10 Emole J A J h s s ν − − − = ⋅ ⋅ = × × × ⋅ × = ×
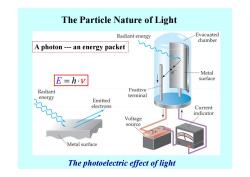
The Particle Nature of Light Radiant energy Evacuated chamber A photon---an energy packet Metal E=h.v surface Radiant Positive energy terminal Emitted electrons Current indicator Voltage source Metal surface The photoelectric effect of light
The Particle Nature of Light E = ⋅ h ν A photon --- an energy packet The photoelectric effect of light
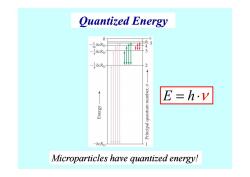
Quantized Energy 0 00 -1hcRH 6 5 16 4 -ghcRu 3 heRH 2 uraqunu wnquenb [edpouud E=h.v K81aug -hcRH 1 Microparticles have quantized energy!
Quantized Energy E h = ⋅ ν Microparticles Microparticles have quantized energy! have quantized energy! E h = ⋅ ν

6.4 Particle-Wave Duality Is light a wave or a particle? Light's wave characteristics-- electromagnetic radiation Light's particle characteristics- based on Einstein's interpretation of the photoelectric effect. E=h.v Particle-wave duality: “photons' It's both a wave and a particle!
6.4 Particle 6.4 Particle-Wave Duality Wave Duality • Light’s wave characteristics -- electromagnetic radiation • Light’s particle characteristics– based on Einstein’s interpretation of the photoelectric effect. Particle Particle-wave duality wave duality : “photons” It’s both a wave and a particle! E = ⋅ h ν
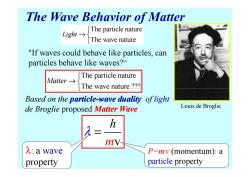
The Wave Behavior of Matter The particle nature Light→ The wave nature "If waves could behave like particles,can particles behave like waves? The particle nature Matter→s The wave nature ?? Based on the particle-wave duality of light de Broglie proposed Matter Wave Louis de Broglie h mV- λ:a wave P=mv(momentum):a property particle property
The Wave Behavior of Matter The particle nature The wave nature Light → The particle nature The wave nature ??? Matter → "If waves could behave like particles, can particles behave like waves?“ Based on the particle particle-wave duality wave duality of light de Broglie proposed Matter Wave The wave nature ??? m v h λ = P=m v (momentum): a particle property λ: a wave property Louis de Broglie
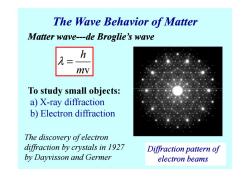
The Wave Behavior of Matter Matter wave---de Broglie's wave h mv To study small objects: a)X-ray diffraction b)Electron diffraction The discovery ofelectron diffraction by crystals in 1927 Diffraction pattern of by Dayvisson and Germer electron beams
The Wave Behavior of Matter v h m λ = Matter wave Matter wave---de Broglie’s wave de Broglie’s wave To study small objects: a) X-ray diffraction b) Electron diffraction Diffraction pattern of electron beams The discovery of electron diffraction by crystals in 1927 by Dayvisson and Germer

Electron diffraction by crystals Diffracled electrons vacuum Electron diffraction rings Electron beam Thin metal foil Salt Crystal Screen
Electron diffraction by crystals Salt Crystal
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nano-scale materials in chemistry.pdf
- 《高分子化学》课程教学资源(参考材料)Lecture Notes in Chemistry Volume 82《Principles of Polymer Design and Synthesis》.pdf
- 《高分子化学》课程教学资源(参考书籍)Paul C. Hiemenz&Timothy P. Lodge《Polymer Chemistry》第二版(Second Edition).pdf
- 《高分子化学》课程教学资源(参考书籍)CHRISTOPHER S.BRAZEL、STEPHEN L.ROSEN《FUNDAMENTAL PRINCIPLES OF POLYMERIC MATERIALS》(Third Edition).pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)自由基聚合(连锁聚合).pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)缩聚和逐步聚合.pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)缩聚和逐步聚合的实施方法.pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)体型缩聚与缩聚共聚.pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)绪论(郭晓霞).pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第四章 太阳能与光伏发电.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第二章 化学电池原理与应用.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第三章 氢能与高分子电解质膜燃料电池(Hydrogen Energy and Polymer Electrolyte Membrane Fuel Cells).pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第一章 能源与高分子概论.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第四章 太阳能与光伏发电.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第五章 风力发电与储能电池.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第二章 化学电池原理与应用.pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第三章 氢能与高分子电解质燃料电池(Hydrogen Energy and Polymer Electrolyte Membrane Fuel Cells).pdf
- 上海交通大学:《清洁能源技术原理与应用》课程教学资源(课件讲义)第一章 能源与高分子概论(主讲:房建华).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.4-9.7).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nanoscale materials in chemistry.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 11 Intermolecular Forces.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)polymers and plastics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(2/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(3/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(1/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.1-6.4).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.5-6.9).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.4-9.6).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.7).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)discussion-organic dyes-color.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Introduction of Chem(刘萍).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 2 Naming Inorganic Compounds.pdf
