上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.7)

Atomic orbital Molecular orbital πbond H H H H o bond
Atomic orbital ? Molecular orbital ?

9.7 Molecular Orbital (MO)Theory 分子轨道理论 What's a MO Atomic Orbitals (AO) Wave functions ( Molecular Orbitals (MO) Different‘shapes': -Molecular Obitals (MO):o(sigma)and (pi)bond -Atomic Orbitals (AO):s,p,d,f,etc
9.7 Molecular Orbital (MO) Theory Atomic Orbitals ( ) Wave functions ( ) Molecular Orbitals ( ) A MO O ψ 分子轨道理论 Different ‘shapes’: – Molecular Obitals (MO): σ (sigma) and π (pi) bond – Atomic Orbitals (AO): s, p, d, f, etc
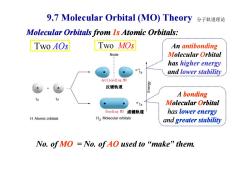
9.7 Molecular Orbital (MO)Theory 分子轨道理论 Molecular Orbitals from Is Atomic Orbitals: Two A0s Two MOs An antibonding Node Molecular Orbital has higher energy s and lower stability Antibonding MO 反键轨道 A bonding 01 Molecular Orbital Bonding MO成键轨道 has lower energy H Atomic orbitals H2 Molecular orbitals and greater stability No.ofMO=No.ofAO used to“make”them
9.7 Molecular Orbital (MO) Theory Two AOs Two MOs An antibonding Molecular Orbital has higher energy and lower stability Molecular Orbitals from 1s Atomic Orbitals: 分子轨道理论 反键轨道 No. of MO = No. of AO used to “make” them. A bonding Molecular Orbital has lower energy and greater stability 反键轨道 成键轨道

Molecular Orbitals in H2 Why H2 is more stable than individual H atoms? Node Antibonding MO K6ieu3 Energy-level diagram(能级图) 015 1s Bonding MO H Atomic orbitals H2 Molecular orbitals 1s 1s H atom H atom 2 bonding electrons keep the two atoms H:molecule together. olecular orbital diagram(分子轨道能力图)
Molecular Orbitals in H2 Why H2 is more stable than individual H atoms? Energy-level diagram level diagram (能级图) 2 bonding electrons keep the two atoms together. Molecular orbital diagram (分子轨道能力图)

9.7 Molecular Orbital (MO)Theory Electron configuration of quantized MO: Lowest energy rule ---Lowest energy level fills first Pauli's Exclusion Principle ---2e maximum per orbital Hund's rule --For degenerate orbitals 1 electron in each orbital before double-up!
9.7 Molecular Orbital (MO) Theory Lowest energy rule --- Lowest energy level fills Lowest energy level fills first Electron configuration of quantized MO : Pauli’s Exclusion Principle --- 2e maximum per 2e maximum per orbital Hund’s rule --- For degenerate orbitals : 1 electron in each orbital before double electron in each orbital before double-up!
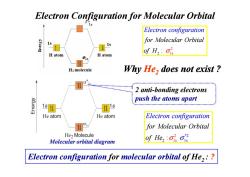
Electron Configuration for Molecular Orbital Electron configuration for Molecular Orbital 1s of H2:ois H atom H atom H:molecule Why He,does not exist 2 anti-bonding electrons K6Jau3 push the atoms apart 1 s He atom He atom Electron configuration for Molecular Orbital He2 Molecule Molecular orbital diagram of Hes Electron configuration for molecular orbital of He,:
Why He2 does not exist ? Electron Configuration for Molecular Orbital 2 anti-bonding electrons 2 2 1s : for Molecular Orbit E a lectron configuration l of H σ 2 anti-bonding electrons push the atoms apart 1 *2 2 1s 2 s : for Molecular Orbit Electron configuratio l f n a o He σ σ Molecular orbital diagram Electron configuration for molecular orbital of He2 : ?
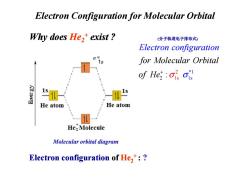
Electron Configuration for Molecular Orbital Why does He,+exist? (分子轨道电子排布式) Electron configuration for Molecular Orbital of He :OisOis He atom He atom He:Molecule Molecular orbital diagram Electron configuration of He,:
Why does He2+ exist ? Electron Configuration for Molecular Orbital 2 1 *1 1s 2 s : for Molecular Orbital of H Electron configuration e σ σ + (分子轨道电子排布式) Electron configuration of He2+ : ? Molecular orbital diagram
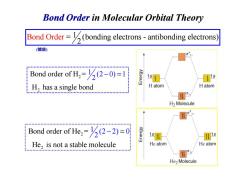
Bond Order in Molecular Orbital Theory Bond Order=(bonding electrons-antibonding electrons (健级) Bond order of H=(2-0)=1 1s s H,has a single bond H atom H atom HHEH.HEM..MNMMM.MN.MMM3an...... H2 Molecule 11 Bond order of He=(2-2)=0 He,is not a stable molecule He atom He atom He>Molecule
Bond Order in Molecular Orbital Theory Bond Ord = (bonding electrons - antibonding 1 electr 2 er ons) 2 2 1 Bond order of H = (2 0) 1 H has a singl 2 e bond − = (键级) 2 H has a single bond 2 2 Bond order of He = He is not a stable 1 (2 m 2) 0 2 olecule − =

Bond Order in Molecular Orbital Theory K3ouH W 01s 01s H吃 H2 He He2 bond 1 0 order; h h (健级)
Bond Order in Molecular Orbital Theory bond order: ½ 1 0 ½ __ __ __ __ (键级)
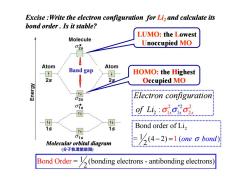
Excise Write the electron configuration for Li,and calculate its bond order.Is it stable? LUMO:the Lowest Molecule 克 Unoccupied MO Atom Band gap Atom HOMO:the Highest 2s 2s Occupied MO Electron configuration of Li: 1s Bond order of Li2 15 Molecular orbital diagram -(4-2)=1 (one a bond) (分子轨道能级图 Bond Order=(bonding electrons-antibonding electrons)
Excise :Write the electron configuration for Li2 and calculate its bond order . Is it stable? LUMO: the Lowest Unoccupied MO HOMO: the Highest Occupied MO Band gap 2 Bond order of Li = (4 2) 1 2 − =1 ( one bond σ ) 2 2 1 2 * 2 2 1 : s s s Electron configuration of Li σ σ σ Bond Ord = (bonding electrons - antibonding 1 electr 2 er ons) Molecular orbital diagram (分子轨道能级图)
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.4-9.6).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.5-6.9).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.1-6.4).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(1/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(3/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(2/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)polymers and plastics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 11 Intermolecular Forces.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nanoscale materials in chemistry.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.4-9.7).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.4-6.9).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nano-scale materials in chemistry.pdf
- 《高分子化学》课程教学资源(参考材料)Lecture Notes in Chemistry Volume 82《Principles of Polymer Design and Synthesis》.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)discussion-organic dyes-color.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Introduction of Chem(刘萍).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 2 Naming Inorganic Compounds.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chromatography-A colarful world.pdf
- 苏州大学化学化工学院:《无机化学》课程教学资源(授课教案,药学、生物制药、中药专业).pdf
- 苏州大学医学部药学院:《生物化学(五)Biochemistry V》课程教学资源(教学大纲).docx
- 苏州大学医学部药学院:《生物化学(五)实验 Experiment of Biochemistry and Molecular Biology》课程教学资源(教学大纲).docx
- 苏州大学化学化工学院:《无机化学 Inorganic chemistry》课程教学资源(教学大纲).docx
- 苏州大学化学化工学院:《无机化学实验 Inorganic Chemistry Experiments》课程教学资源(教学大纲,药学类专业).docx
- 长春理工大学化学与环境工程学院:教学大纲合集(学科基础课程、专业教育课程、大光电课程、基础实践课程、专业实践课程、综合实践课程).pdf
- 中国科学技术大学:波色系统(PPT讲稿)超流性.ppt
- 东莞理工学院:《循环经济与可持续发展》课程教学资源(教学大纲)程可可-化卓.docx
- 东莞理工学院:《循环经济与可持续发展》课程教学资源(教学大纲)程可可-应化.docx
- 东莞理工学院:2022-2023 第一学期《功能高分子材料》课程教学资源(教学大纲)赵莉丽-19应用化学,化卓班.docx
- 东莞理工学院:2022-2023学年第一学期《锂离子电池工程思维与方法》课程教学资源(教学大纲)赵丽源(1).docx
- 东莞理工学院:2022-2023学年第一学期《无机化学》课程教学资源(教学大纲)李超-2022级应化1、2班-教学大纲.docx
- 东莞理工学院:2022-2023学年第一学期《无机化学》课程教学资源(教学大纲)王永东-2022级应化卓越1、2班-教学大纲.docx
- 东莞理工学院:2022-2023学年第一学期《物理化学》课程教学资源(教学大纲)2020级应用化学1、2班-宋金刚 苗荣荣-教学大纲-20220826.docx
- 东莞理工学院:2022-2023学年第一学期《物理化学》课程教学资源(教学大纲)2021级食品科学与工程1班-宋金刚-教学大纲-20220826.docx
