上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.1-6.4)

Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter 6.5 Quantum Mechanical Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations Periodic Table Chapter 6
Chapter 6 Electronic Structure of Atoms 6.4 The Wave Behavior of Matter Chapter 6 6.5 Quantum Mechanical & Atomic Orbitals 6.6 Representations of Orbitals 6.7 Many electron atoms 6.8 Electron Configurations 6.9 Electron Configurations & Periodic Table
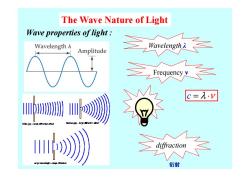
The Wave Nature of Light Wave properties of light Wavelength A Amplitude kcue c=A.v Wide gop-small efec 山》 衍射
The Wave Nature of Light Wave properties of light : Wavelength λ Frequency ν diffraction c = ⋅ λ ν 衍射
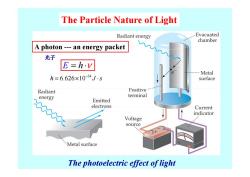
The Particle Nature of Light Radiant energy Evacuated chamber A photon---an energy packet 光子 E=h.v Metal h=6.626×10-34J-s surface Radiant Positive energy terminal Emitted electrons Current indicator Voltage source Metal surface The photoelectric effect of light
The Particle Nature of Light E = ⋅ h ν A photon --- an energy packet 光子 34 h J s 6.626 10 − = × ⋅ The photoelectric effect of light

Duality of Light A photon--- an energy packet Wavelength E hoton =h.v h=6.626×10-34Js =A.h.v 乏diffraction≤ A=6.02×1023/mol The Particle The Wave Nature of Light Nature of Light
Duality of Light Wavelength λ Frequency ν A photon --- an energy packet Ephoton = ⋅ h ν 34 h J s 6.626 10− = × ⋅ The Wave Nature of Light The Particle Nature of Light diffraction ν mole E = ⋅ ⋅ A h ν 23 A mol = × 6.02 10 / 34 h J s 6.626 10− = × ⋅
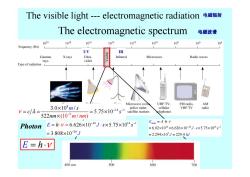
The visible light---electromagnetic radiation 电磁辐射 The electromagnetic spectrum 电磁波谱 1020 1018 1016 104 1012 1010 10 105 104 Frequency (Hz) UV IR Gamma X rays Ultra- Infrared Microwave Radio waves rays violet Type of radiation L Microwave ovel UHF TV, FM radio. AM 3.0×108m/s police radar. VHF TV V=c/A= =5.75x10-14s-satellite stations cellular radio elephones 522nm×(10-°m/nm) E=hy=6.626×10-34J·s×5.75×1014s1 Emole=A.h-v Photon =6.02×1023×6.626×10-34Js×5.75×104s =3.808×10-20J =2.294×103J=229.4kJ E=h.v 400nm 500 600 700
The electromagnetic spectrum The visible light --- electromagnetic radiation 电磁辐射 电磁波谱 UV IR E = ⋅ h ν Photon 34 14 1 20 6.626 10 5.75 10 3.808 10 E h s J s J ν − − − = ⋅ × × × = × = ⋅ 23 34 14 1 5 6.02 10 6.626 10 5.75 10 2.294 10 229.4 Emole J A s kJ s J h ν − − = ⋅ ⋅ = × × × ⋅ × × = × = 8 14 1 9 (1 3.0 10 / 5.75 10 522 0 / ) m s c n m n s m m ν λ − − × − = = = × ×

6.4 Particle-Wave Duality Is light a wave or a particle? Light's wave characteristics-- electromagnetic radiation Light's particle characteristics- based on Einstein's interpretation of the photoelectric effect. E=h.v Particle-wave duality: “photons' It's both a wave and a particle!
6.4 Particle 6.4 Particle-Wave Duality Wave Duality • Light’s wave characteristics -- electromagnetic radiation • Light’s particle characteristics– based on Einstein’s interpretation of the photoelectric effect. Particle Particle-wave duality wave duality : “photons” It’s both a wave and a particle! E = ⋅ h ν
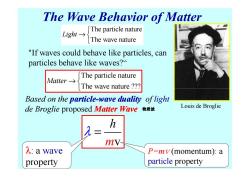
The Wave Behavior of Matter The particle nature Light→ The wave nature "If waves could behave like particles,can particles behave like waves? The particle nature Aatter→s The wave nature ?? Based on the particle-wave duality of light de Broglie proposed Matter Wave物质被 Louis de Broglie h mV- λ:a wave P=mv(momentum):a property particle property
The Wave Behavior of Matter The particle nature The wave nature Light → The particle nature The wave nature ??? Matter → "If waves could behave like particles, can particles behave like waves?“ Based on the particle particle-wave duality wave duality of light de Broglie proposed Matter Wave The wave nature ??? m v h λ = P=m v (momentum): a particle property λ: a wave property Louis de Broglie 物质波
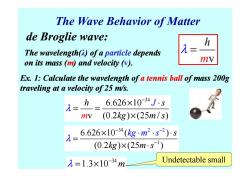
The Wave Behavior of Matter de Broglie wave: h The wavelength(A)of a particle depends on its mass (m)and velocity (v). mV Ex.1:Calculate the wavelength of a tennis ball of mass 200g traveling at a velocity of 25 m/s. h 6.626×10-34Js = mv (0.2kg)×(25m/s) 6.626×10-34(kg·m2.s2)s (0.2kg)×(25m·s) 元=1.3×10-34m Undetectable small
The Wave Behavior of Matter Ex. 1: Calculate the wavelength of a tennis ball of mass 200g traveling at a velocity of 25 m/s. m v h λ = de Broglie wave: The wavelength(λ) of a particle depends on its mass (m) and velocity (v). traveling at a velocity of 25 m/s. 34 6.626 10 v (0.2 ) (25 / ) h s J m kg m s λ − × ⋅ = = × 1 34 2 2 6.626 10 ( ) (0.2 ) (25 ) s kg m s kg m s λ − − − × ⋅ = × ⋅ ⋅ ⋅ 34 λ 1.3 10 m − = × Undetectable small
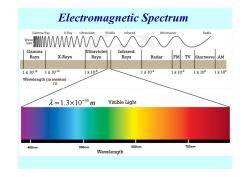
Electromagnetic Spectrum Gamma Ray X-Ray Ultraviolet Visible Infrared Microwave Radio Wave Type WWAA/ Gamma Ultraviolet Infrared Rays X-Rays Rays Rays Radar FM V Shortwave AM 1x1014 1×1012 1x10-8 1x104 1×102 1×102 1x104 Wavelength(in meters) m 元=1.3×10-34m Visible Light 400nm 500nm 600nm 700nm Wavelength
Electromagnetic Spectrum m 34 λ 1.3 10 m − = ×

The Wave Behavior of Matter de Broglie wave: h Power to:升幂 = mv Ex.2:Calculate the wavelength of an electron with mass of 9.0x10-31 kg traveling at 2/3 the speed of light (3.0x108 m/s) h 6.626×10-34J5 λ= mv (9.1x10kg)x3×3.0×10m/s) 6.626×10-34(kgm2.82)s (9.1×1031kg)×(2.0×108m/s) 2=3.64×10-12m=0.00364m ---X ray
The Wave Behavior of Matter Ex.2: Calculate the wavelength of an electron with mass of 9.0×10-31 kg traveling at 2/3 the speed of light (3.0×10 8 m/s) m v h de Broglie wave: λ = 34 h s 6.626 10 J λ − × ⋅ = = Power to:升幂 31 8 6.626 10 v 2 (9.1 10 ) ( 3.0 10 / ) 3 h s kg J m m s λ − × ⋅ = = × × × × 34 3 2 2 1 8 6.626 10 ( ) (9.1 10 ) (2.0 10 / ) s kg m m s s kg λ − − − × ⋅ = × × × ⋅ ⋅ 12 λ 3.64 10 0.00364 m nm − = × = ---X ray
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(1/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(3/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics(2/3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)polymers and plastics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 11 Intermolecular Forces.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nanoscale materials in chemistry.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.4-9.7).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.4-6.9).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap.20 Voltaic Cells(Galvanic Cells).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 19 Chemical Thermodynamics.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Nano-scale materials in chemistry.pdf
- 《高分子化学》课程教学资源(参考材料)Lecture Notes in Chemistry Volume 82《Principles of Polymer Design and Synthesis》.pdf
- 《高分子化学》课程教学资源(参考书籍)Paul C. Hiemenz&Timothy P. Lodge《Polymer Chemistry》第二版(Second Edition).pdf
- 《高分子化学》课程教学资源(参考书籍)CHRISTOPHER S.BRAZEL、STEPHEN L.ROSEN《FUNDAMENTAL PRINCIPLES OF POLYMERIC MATERIALS》(Third Edition).pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)自由基聚合(连锁聚合).pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)缩聚和逐步聚合.pdf
- 上海交通大学:《高分子化学 Polymer Chemistry》课程教学资源(课件讲稿)缩聚和逐步聚合的实施方法.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 6 Electronic Structure of Atoms(6.5-6.9).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 8 Basic Concepts of Chemical Bonding.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.1-9.3).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.4-9.6).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories(9.7).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chap. 9 Molecular Geometry and Bonding Theories.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)discussion-organic dyes-color.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Introduction of Chem(刘萍).pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chapter 2 Naming Inorganic Compounds.pdf
- 上海交通大学:《大学化学 Chemistry》教学资源(课件讲稿)Chromatography-A colarful world.pdf
- 苏州大学化学化工学院:《无机化学》课程教学资源(授课教案,药学、生物制药、中药专业).pdf
- 苏州大学医学部药学院:《生物化学(五)Biochemistry V》课程教学资源(教学大纲).docx
- 苏州大学医学部药学院:《生物化学(五)实验 Experiment of Biochemistry and Molecular Biology》课程教学资源(教学大纲).docx
- 苏州大学化学化工学院:《无机化学 Inorganic chemistry》课程教学资源(教学大纲).docx
- 苏州大学化学化工学院:《无机化学实验 Inorganic Chemistry Experiments》课程教学资源(教学大纲,药学类专业).docx
- 长春理工大学化学与环境工程学院:教学大纲合集(学科基础课程、专业教育课程、大光电课程、基础实践课程、专业实践课程、综合实践课程).pdf
- 中国科学技术大学:波色系统(PPT讲稿)超流性.ppt
- 东莞理工学院:《循环经济与可持续发展》课程教学资源(教学大纲)程可可-化卓.docx
- 东莞理工学院:《循环经济与可持续发展》课程教学资源(教学大纲)程可可-应化.docx
- 东莞理工学院:2022-2023 第一学期《功能高分子材料》课程教学资源(教学大纲)赵莉丽-19应用化学,化卓班.docx
