《化工热力学》课程授课教案(讲义)Chapter 8 Production of Power from Heat

Chapter 8 Production of Power from Heat content 8.1 The steam power plant 8.2 Internal-Combustion Engines 8.3 Jet Engines;Rocket Engines
Chapter 8 Production of Power from Heat content 8.1 The steam power plant 8.2 Internal-Combustion Engines 8.3 Jet Engines; Rocket Engines

Introduction Forms ofenergy used by mankind Energy from the sun The kineticenergy associated with atmospheric winds The potential energy oftides Hydroelectric power Chemical energy of fuels combustion fission Heat Nuclear energy Heat engine the steam powerplant the internal-combustion Mechanical engine energy This chapter is devoted to the analysis of several common heat-engine cycles 目的: 研究循环中热、功转换的效果及其影响因素,探求提高能量转换 效果的途径。 内容: 讨论蒸汽动力循环的热效率、功以及循环中各工质状态的变化。 要求:
Introduction Forms of energy used by mankind Energy from the sun The kinetic energy associated with atmospheric winds The potential energy of tides Hydroelectric power This chapter is devoted to the analysis of several common heat-engine cycles 目的: 研究循环中热、功转换的效果及其影响因素,探求提高能量转换 效果的途径。 内容: 讨论蒸汽动力循环的热效率、功以及循环中各工质状态的变化。 要求: Heat Mechanical energy Chemical energy of fuels Nuclear energy the steam power plant the internal-combustion engine Heat engine combustion fission
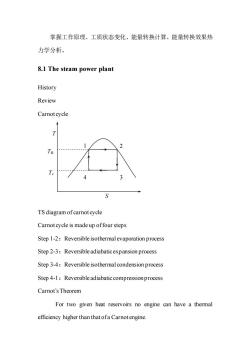
掌握工作原理、工质状态变化、能量转换计算、能量转换效果热 力学分析。 8.1 The steam power plant History Review Carnotcycle TH T TS diagram of carnot cycle Carnot cycle is made up offour steps Step 1-2:Reversible isothermal evaporation process Step 2-3:Reversibleadiabaticexpansion process Step 3-4:Reversible isothermal condension process Step 4-1:Reversible adiabatic compressionprocess Carnot's Theorem For two given heat reservoirs no engine can have a thermal efficiency higher than that ofa Carnot engine
掌握工作原理、工质状态变化、能量转换计算、能量转换效果热 力学分析。 8.1 The steam power plant History Review Carnot cycle TS diagram of carnot cycle Carnot cycle is made up of four steps Step 1-2:Reversible isothermal evaporation process Step 2-3:Reversible adiabatic expansion process Step 3-4:Reversible isothermal condension process Step 4-1:Reversible adiabatic compression process Carnot’s Theorem For two given heat reservoirs no engine can have a thermal efficiency higher than that of a Carnot engine. 3 1 2 4 T S TH Tc
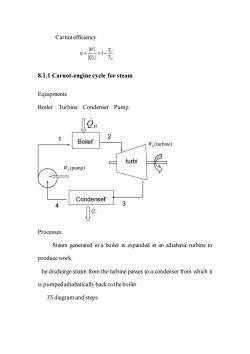
Carnot efficiency 卧1 8.1.1 Carnot-engine cycle for steam Equipments Boiler Turbine Condenser Pump: 0⑨H 2 Boiler Ws(turbine) turbi 市,(pump) Condenser 3 Processes Steam generated in a boiler is expanded in an adiabatic turbine to produce work. he discharge steam from the turbine passes to a condenser from which it is pumped adiabatically back to the boiler TS diagram and steps
Carnot efficiency 1 C H H W T Q T = = − 8.1.1 Carnot-engine cycle for steam Equipments Boiler Turbine Condenser Pump: Processes Steam generated in a boiler is expanded in an adiabatic turbine to produce work. The discharge steam from the turbine passes to a condenser from which it is pumped adiabatically back to the boiler. TS diagram and steps Boiler Condenser turbi ne QH (turbine) WS QC (pump) WS 3 1 4 2

TH Tc S Step 1-2:Isothermal evaporationprocess in the boiler Step 2-3:Reversible adiabatic expansionprocess in the turbine Step 3-4:Isothermal partial condension in condenser Step 4-1:Reversible adiabatic compression process by the pump Analysis for whole system AH+号+gm于0+形 0 0+巾,=0 序,=m,(turbine))-f,(pmp) el=0-loc W,=W.(turbine)-W.(pump)=Qu-Qc Efficiency Practical difficulties attend the operation ofequipment (1)湿蒸汽对汽轮机有侵蚀作用:
Step 1-2: Isothermal evaporation process in the boiler Step 2-3: Reversible adiabatic expansion process in the turbine Step 3-4: Isothermal partial condension in condenser Step 4-1: Reversible adiabatic compression process by the pump Analysis for whole system 2 ( ) 2 s u + + = + H gz m Q W 0 Q W+ = s ( ) ( ) W W turbine W pump s s s = − Q Q Q = − H C ( ) ( ) W W turbine W pump Q Q s s s H C = − = − Efficiency 1 C H H W T Q T = = − Practical difficulties attend the operation of equipment (1)湿蒸汽对汽轮机有侵蚀作用; 1 4 T S 3 2 TC TH 0
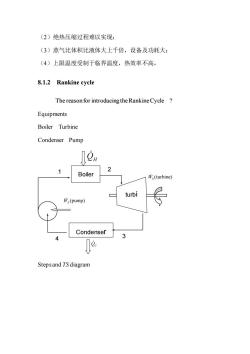
(2)绝热压缩过程难以实现: (3)蒸气比体积比液体大上千倍,设备及功耗大: (4)上限温度受制于临界温度,热效率不高。 8.1.2 Rankine cycle The reason for introducing the Rankine Cycle Equipments Boiler Turbine Condenser Pump 00H 7 2 Boiler 序,(turbine) turbi ∥(pump) Condenser 3 Stepsand TSdiagram
(2)绝热压缩过程难以实现; (3)蒸气比体积比液体大上千倍,设备及功耗大; (4)上限温度受制于临界温度,热效率不高。 8.1.2 Rankine cycle The reason for introducing theRankineCycle ? Equipments Boiler Turbine Condenser Pump Steps and TS diagram Boiler Condenser turbi ne QH (turbine) WS QC (pump) WS 3 1 4 2
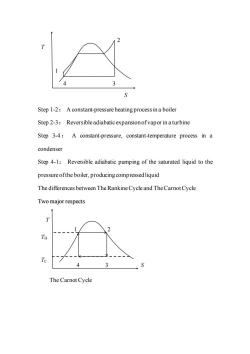
Step 1-2:A constant-pressure heatingprocess ina boiler Step 2-3:Reversible adiabaticexpansionofvapor inaturbine Step 3-4:A constant-pressure,constant-temperature process in a condenser Step 4-1:Reversible adiabatic pumping of the saturated liquid to the pressure ofthe boiler,producing compressed liquid The differences between The Rankine Cycle and The Carnot Cycle Two major respects T TH Te 4 The Carnot Cycle
Step 1-2: A constant-pressure heating process in a boiler Step 2-3: Reversible adiabatic expansion of vapor in a turbine Step 3-4: A constant-pressure, constant-temperature process in a condenser Step 4-1: Reversible adiabatic pumping of the saturated liquid to the pressure of the boiler, producing compressed liquid The differences between The Rankine Cycle and The Carnot Cycle Two major respects The Carnot Cycle T S 1 4 3 2 T S 1 4 3 2 TC TH
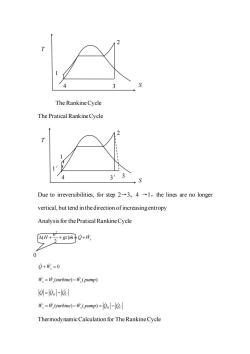
The Rankine Cycle The Pratical Rankine Cycle 33 S Due to irreversibilities,for step 2-3,4-1,the lines are no longer vertical,but tend in the direction of increasingentropy Analysis for the Pratical Rankine Cycle 1+g网e+晚 0 Q+巾,=0 W,=W,(turbine)-W,(pump) lol=l0.-lecl W,=W,(turbine)-W,(pump)=On-Qc Thermodynamic Calculation for The Rankine Cycle
The Rankine Cycle The Pratical Rankine Cycle Due to irreversibilities, for step 2→3,4 →1,the lines are no longer vertical, but tend in the direction of increasing entropy Analysis for the Pratical Rankine Cycle 2 ( ) 2 s u + + = + H gz m Q W 0 Q W+ = s ( ) ( ) W W turbine W pump s s s = − Q Q Q = − H C ( ) ( ) W W turbine W pump Q Q s s s H C = − = − Thermodynamic Calculation for The Rankine Cycle T S 1 4 3 2 1 4 1′ S T 3′ 3 2 0
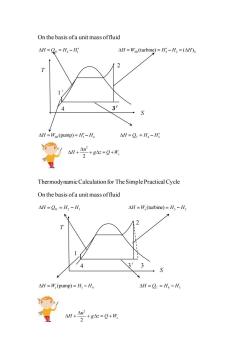
On the basis ofa unit mass offluid AH=Q=H2-Hi △H=Wx(turbine)=H;-H2=(△H)s 1 3 △H=W(pump)=H-H, △H=Q=H,-Hg 4H+42 +g△=Q+W 2 Thermodynamic Calculation for The Simple Practical Cycle On the basis ofa unit mass offluid △H=QH=H2-H, AH=W.(turbine)=H-H 7 △H=W,(pump)=H,-H △H=Q=H,-H 4H+4 2+84=Q+形
On the basis of a unit mass of fluid H Q H H H 2 1 = = − 3 2 (turbine) ( ) H W H H H SR S = = − = 1 4 (pump) H W H H SR = = − H Q H H C 4 3 = = − 2 2 s u H g z Q W + + = + Thermodynamic Calculation for The Simple Practical Cycle On the basis of a unit mass of fluid = = − H Q H H H 2 1 3 2 (turbine) = = − H W H H s 1 4 (pump) = = − H W H H s = = − H Q H H C 4 3 2 2 s u H g z Q W + + = + T S 1′ 4 3′ 2 T S 1 4 3′ 2 3
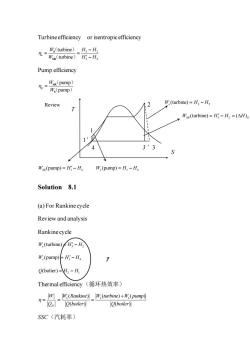
Turbine efficiency or isentropic efficiency 品-款气受 Pump efficiency 哥 Review W,(turbine)=H3H T Wsg(turbine)=HH=(AH) 33 Wse(pump)=HH W.(pump)=H Solution 8.1 (a)For Rankinecycle Review and analysis Rankinecycle W,(turbine)H W,(pump)H Q(bolier)2-H Thermal efficiency(循环热效率) WW(Rankine)W(turbine)+W.(pump) n(boiler) O(boiler) SSC(汽耗率)
Turbine efficiency or isentropic efficiency 3 2 3 2 turbine turbine W H H W H H − = = − S s SR ( ) ( ) Pump efficiency p pump pump W W = SR S ( ) ( ) 3 2 (turbine) W H H s = − 3 2 (turbine) ( ) W H H H SR S = − = 1 4 (pump) W H H SR = − 1 4 (pump) W H H s = − Solution 8.1 (a) For Rankine cycle Review and analysis Rankine cycle 3 2 (turbine) W H H s = − 1 4 (pump) W H H s = − 2 1 Q H H (bolier) = − Thermal efficiency(循环热效率) H W Q = s ( ) ( ) W Rankine Q boiler = s s ( ) ( ) ( ) W turbine W pump Q boiler + = SSC(汽耗率) T S 1 4 3′ 2 1′ 3 Review ?
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《化工热力学》课程授课教案(讲义)Chapter 7 Application of Thermodynamics to Flow Processes.doc
- 《化工热力学》课程授课教案(讲义)Chapter 6 Thermodynamic properties of fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 5 The Second Law of Thermodynamics.doc
- 《化工热力学》课程授课教案(讲义)Chapter 3 Volumetric Properties of Pure Fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 2 The First Law and Other Basic Concepts.doc
- 《化工热力学》课程教学大纲 Thermodynamics of chemical engineering(新疆大学:黄雪莉).doc
- 海南大学:《精细化学品与工艺学》课程授课教案(讲义,共十章,负责人:刘钟馨、韩秀萍).docx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)食品添加剂 Food additives.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(醇酸树脂涂料、丙烯酸树脂涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)表面活性剂 surfactants.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)合成材料助剂.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)胶黏剂.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)绪论.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(环氧树脂涂料、聚氨酯树脂涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(涂料的概念、作用和组成).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料分类(涂料的分类和命名、溶剂型涂料、水性涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)有机染料和颜料.pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)化妆品.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)香料.ppt
- 海南大学:《精细化学品与工艺学》课程教学资源(参考资料)冠状病毒知识梳理.pptx
- 《化工热力学》课程授课教案(讲义)Chapter 9 Refrigeration and Liquefaction.doc
- 《化工热力学》课程授课教案(讲义)Chapter 11 Solution thermodynamics - Theory.doc
- 《化工热力学》课程授课教案(讲义)Chapter 12 Solution Thermodynamics:Application.doc
- 《化工热力学》课程授课教案(讲义)Chapter 13 Chemical-Reaction Equilibria.doc
- 《化工热力学》课程教学资源(作业习题)第1章 绪言(无答案).doc
- 《化工热力学》课程教学资源(作业习题)第2章 流体的P-V-T关系(含答案).doc
- 《化工热力学》课程教学资源(作业习题)第4章 非均相封闭体系热力学(选择题带答案).doc
- 《化工热力学》课程教学资源(作业习题)第5章 非均相体系热力学性质计算(无答案).doc
- 《化工热力学》课程教学资源(作业习题)第8章 化学反应平衡(含答案).doc
- 《化工热力学》课程教学课件(英文讲稿)纯流体性质 Volumetric Properties of Pure Fluids.pdf
- 《化工热力学》课程教学课件(英文讲稿)流体热力学性质 Thermodynamic properties of fluids.pdf
- 《化工热力学》课程教学课件(英文讲稿)热动力 Production of Power from Heat.pdf
- 《化工热力学》课程教学课件(英文讲稿)溶液热力学原理 Solution thermodynamics:Theory.pdf
- 《化工热力学》课程教学课件(英文讲稿)热功转换及可用能.pdf
- 《化工热力学》课程教学课件(PPT讲稿)第一章 绪论 Chemical Engineering thermodynamics.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第二章 流体的p-V-T关系.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第五章 化工过程的能量分析.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第七章 相平衡.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第三章 流体的热力学性质.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第六章 蒸汽动力循环与制冷循环.ppt
