《化工热力学》课程教学课件(英文讲稿)流体热力学性质 Thermodynamic properties of fluids

Chapter 6 Thermodynamic properties of fluids
Chapter 6 Thermodynamic properties of fluids

6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 6.3 Residual Properties by Equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases
6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 6.3 Residual Properties by Equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases
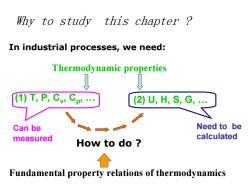
Why to study this chapter? In industrial processes,we need: Thermodynamic properties T,P,Cv:Cp' (2)U,H,S,G Can be Need to be measured calculated How to do Fundamental property relations of thermodynamics
Why to study this chapter ? In industrial processes, we need: Thermodynamic properties (1) T, P, C v, C p, . (2) U, H, S, G, . How to do ? Can be measured Need to be calculated Fundamental property relations of thermodynamics

·学习化工热力学的目的在于应用,主要的应用就是热 力学性质的推算。 ·本章的主要任务就是将纯物质和均相定组成混合物系 统的一些有用的热力学性质表达成为能够直接测定的p、 V、T及C,*(理想气体热容)的普遍化函数,再结合 状态方程和C,*模型,就可以得到从p、V、T推算其它 热力学性质的具体关系式。即可以实现由一个状态方 程和理想气体热容模型推算其它热力学性质
• 学习化工热力学的目的在于应用,主要的应用就是热 力学性质的推算。 • 本章的主要任务就是将纯物质和均相定组成混合物系 统的一些有用的热力学性质表达成为能够直接测定的p、 V、T及Cp*(理想气体热容)的普遍化函数,再结合 状态方程和Cp*模型,就可以得到从p、V、T推算其它 热力学性质的具体关系式。即可以实现由一个状态方 程和理想气体热容模型推算其它热力学性质

6 Thermodynamic properties of fluids 6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 6.3 Residual Properties by Equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases
6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 6.3 Residual Properties by Equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases 6 Thermodynamic properties of fluids
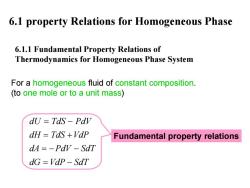
6.1 property Relations for Homogeneous Phase 6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition. (to one mole or to a unit mass) dU Tds-Pdv dH TdS +VdP Fundamental property relations dA=-Pdv-SdT dG=VdP-SdT
6.1 property Relations for Homogeneous Phase 6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition. (to one mole or to a unit mass ) dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + =− − = − Fundamental property relations
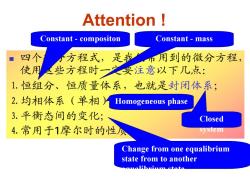
Attention Constant-compositon Constant-mass 四个 小方程式,是我 用到的微分方程, 使用些方程时之安注意以下几点: 1.恒组分、恒质量体系,也就是封闭体系; 2.均相体系(单相1 Homogeneous phase 3.平衡态间的变化; Closed 4.常用于1摩尔时的性顶 system Change from one equalibrium state from to another cumoHhrium stoto
Attention ! Constant - compositon Homogeneous phase Closed system Constant - mass Change from one equalibrium state from to another equalibrium state
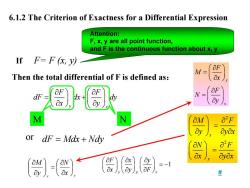
6.1.2 The Criterion of Exactness for a Differential Expression Attention: F,x,y are all point function, and F is the continuous function about x,y IfF=F(,y以 M= Then the total differential of F is defined as: &x OF dF ay M N OM Oyox or dF=Mdx+Ndy O"F ayox =-1
6.1.2 The Criterion of Exactness for a Differential Expression If F= F (x, y) y x F F dF dx dy x y ⎛ ⎞ ∂ ∂⎛ ⎞ = + ⎜ ⎟ ⎜ ⎟ ⎝ ⎠ ∂ ∂ ⎝ ⎠ dF Mdx Nd = + y Then the total differential of F is defined as: or 2 2 x y M F y y x N F x y x ⎛ ⎞ ∂ ∂ ⎜ ⎟ = ⎝ ⎠ ∂ ∂ ∂ ⎛ ⎞ ∂ ∂ ⎜ ⎟ = ⎝ ⎠ ∂ ∂ ∂ x y x N y M ⎟ ⎠ ⎞ ⎜ ⎝ ⎛ ∂ ∂ = ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ ∂ ∂ y x F M x F N y ⎛ ⎞ ∂ = ⎜ ⎟ ⎝ ⎠ ∂ ⎛ ⎞ ∂ = ⎜ ⎟ ⎝ ⎠ ∂ M N 1 y x F Fx y xyF ⎛⎞ ⎛⎞ ∂∂∂ ⎛ ⎞ ⎜⎟ ⎜⎟ ⎜ ⎟ = − ⎝⎠ ⎝⎠ ∂∂∂ ⎝ ⎠ Attention: F, x, y are all point function, and F is the continuous function about x, y #
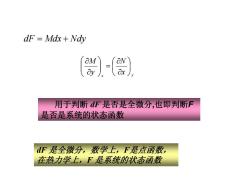
dF =Mdx Ndy aN 用于判断dF是否是全微分,也即判断F 是否是系统的状态函数 dF是全微分,数学上,F是点函数, 在热力学上,F是系统的状态函数
x y x N y M ⎟⎠⎞ ⎜⎝⎛ ∂∂ = ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ dF Mdx Nd = + y 用于判断 dF 是否是全微分,也即判断F 是否是系统的状态函数 dF 是全微分,数学上,F是点函数, 在热力学上,F 是系统的状态函数
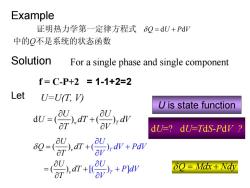
Example 证明热力学第一定律方程式6Q=dU+PdV 中的Q不是系统的状态函数 Solution For a single phase and single component f=C-P+2=1-1+2=2 Let U=U(T,V) U is state function -p. dU-?dU=TdS-Pdy d7+( U aU δQ=Mdx+Wdy
Example 证明热力学第一定律方程式 δQ U PV = d d + 中的Q不是系统的状态函数 Solution For a single phase and single component f = C-P+2 = 1-1+2=2 Let U=U(T, V) d () () v T U U U dT dV T V ∂ ∂ = + ∂ ∂ ( ) [( ) ( ) ] ) ( v T v T U dV PdV U Q dT T U dT V U d T P V V δ ∂ + ∂ ∂ + ∂ = + ∂ ∂ + ∂ = ∂ U is state function δQ Mdx Nd = + y dU=? dU=TdS-PdV ?
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《化工热力学》课程教学课件(英文讲稿)纯流体性质 Volumetric Properties of Pure Fluids.pdf
- 《化工热力学》课程教学资源(作业习题)第8章 化学反应平衡(含答案).doc
- 《化工热力学》课程教学资源(作业习题)第5章 非均相体系热力学性质计算(无答案).doc
- 《化工热力学》课程教学资源(作业习题)第4章 非均相封闭体系热力学(选择题带答案).doc
- 《化工热力学》课程教学资源(作业习题)第2章 流体的P-V-T关系(含答案).doc
- 《化工热力学》课程教学资源(作业习题)第1章 绪言(无答案).doc
- 《化工热力学》课程授课教案(讲义)Chapter 13 Chemical-Reaction Equilibria.doc
- 《化工热力学》课程授课教案(讲义)Chapter 12 Solution Thermodynamics:Application.doc
- 《化工热力学》课程授课教案(讲义)Chapter 11 Solution thermodynamics - Theory.doc
- 《化工热力学》课程授课教案(讲义)Chapter 9 Refrigeration and Liquefaction.doc
- 《化工热力学》课程授课教案(讲义)Chapter 8 Production of Power from Heat.doc
- 《化工热力学》课程授课教案(讲义)Chapter 7 Application of Thermodynamics to Flow Processes.doc
- 《化工热力学》课程授课教案(讲义)Chapter 6 Thermodynamic properties of fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 5 The Second Law of Thermodynamics.doc
- 《化工热力学》课程授课教案(讲义)Chapter 3 Volumetric Properties of Pure Fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 2 The First Law and Other Basic Concepts.doc
- 《化工热力学》课程教学大纲 Thermodynamics of chemical engineering(新疆大学:黄雪莉).doc
- 海南大学:《精细化学品与工艺学》课程授课教案(讲义,共十章,负责人:刘钟馨、韩秀萍).docx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)食品添加剂 Food additives.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(醇酸树脂涂料、丙烯酸树脂涂料).pptx
- 《化工热力学》课程教学课件(英文讲稿)热动力 Production of Power from Heat.pdf
- 《化工热力学》课程教学课件(英文讲稿)溶液热力学原理 Solution thermodynamics:Theory.pdf
- 《化工热力学》课程教学课件(英文讲稿)热功转换及可用能.pdf
- 《化工热力学》课程教学课件(PPT讲稿)第一章 绪论 Chemical Engineering thermodynamics.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第二章 流体的p-V-T关系.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第五章 化工过程的能量分析.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第七章 相平衡.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第三章 流体的热力学性质.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第六章 蒸汽动力循环与制冷循环.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第十章 化学反应平衡.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第四章 流体混合物的热力学性质.ppt
- 《化工原理》课程教学大纲 Principles of Chemical Engineering B.pdf
- 《化学反应工程》课程教学标准B.doc
- 山东理工大学:《化学反应工程》课教学大纲B.doc
- 《化工安全与环保》课程教学资源(课件讲稿)物质性质、物化原理与安全.pdf
- 《化学反应工程》课程教学资源(PPT课件)第3章 釜式及均相管式反应器.ppt
- 《化学反应工程》课程教学资源(PPT课件)第4章 反应器中的混合及对反应的影响.ppt
- 《化学反应工程》课程教学资源(PPT课件)第5章 固定床气-固相催化反应工程.ppt
- 《化学反应工程》课程教学资源(课件讲稿)第2章 气-固相催化反应本征及宏观动力学.pdf
- 《化学反应工程》课程教学资源(PPT课件)第1章 应用化学反应动力学.ppt
