《化工热力学》课程授课教案(讲义)Chapter 9 Refrigeration and Liquefaction
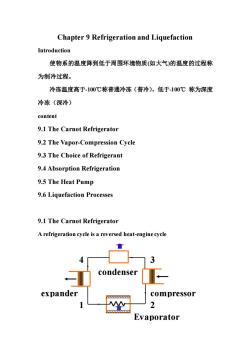
Chapter 9 Refrigeration and Liquefaction Introduction 使物系的温度降到低于周围环境物质(如大气)的温度的过程称 为制冷过程。 冷冻温度高于100℃称普通冷冻(普冷),低于-100℃称为深度 冷冻(深冷) content 9.1 The Carnot Refrigerator 9.2 The Vapor-Compression Cycle 9.3 The Choice of Refrigerant 9.4 Absorption Refrigeration 9.5 The Heat Pump 9.6 Liquefaction Processes 9.1 The Carnot Refrigerator A refrigeration cycle is a reversed heat-engine cycle 音 3 condenser expander compressor 2 Evaporator
Chapter 9 Refrigeration and Liquefaction Introduction 使物系的温度降到低于周围环境物质(如大气)的温度的过程称 为制冷过程。 冷冻温度高于-100℃称普通冷冻(普冷),低于-100℃ 称为深度 冷冻(深冷) content 9.1 The Carnot Refrigerator 9.2 The Vapor-Compression Cycle 9.3 The Choice of Refrigerant 9.4 Absorption Refrigeration 9.5 The Heat Pump 9.6 Liquefaction Processes 9.1 The Carnot Refrigerator A refrigeration cycle is a reversed heat-engine cycle expander compressor 2 4 3 1 condenser Evaporator
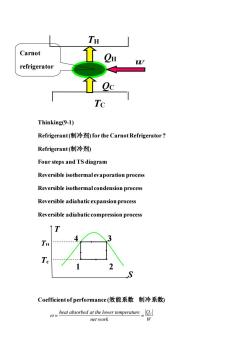
TH Carnot refrigerator Tc Thinking(9-1) Refrigerant(制冷剂for the Carnot Refrigerator? Refrigerant(制冷剂) Four steps and TS diagram Reversible isothermal evaporation process Reversible isothermal condension process Reversible adiabatic expansion process Reversible adiabatic compression process ↑T S Coefficient of performance(效能系数制冷系数) heat absorbed at the lower temperature net work
Thinking(9-1) Refrigerant (制冷剂) for the Carnot Refrigerator ? Refrigerant (制冷剂) Four steps and TS diagram Reversible isothermal evaporation process Reversible isothermal condension process Reversible adiabatic expansion process Reversible adiabatic compression process Coefficient of performance (效能系数 制冷系数) heat absorbed at the lower temperature QC net work W = = TH TC QH QC W 2 4 3 1 T S TH Tc Carnot refrigerator refrigera tor

The cycle requires the addition of net work w=l№al-leal 岛受1经 T Review 2=T(S2-S) Te 1 0. S n=T(S;-S.) 9.2 The Vapor-compression Cycle Plant Evaporator Throttle valve Condenser Compressor
The cycle requires the addition of net work W Q Q = − H C 1 H C C W Q Q Q = − 1 H H C C C C W T T T Q T T − = − = Review QC 2 1 ( ) Q T S S C C = − QH 3 4 ( ) Q T S S H H = − 9.2 The Vapor-compression Cycle Plant Evaporator Throttle valve Condenser Compressor 2 4 3 1 T S TH Tc QC QH
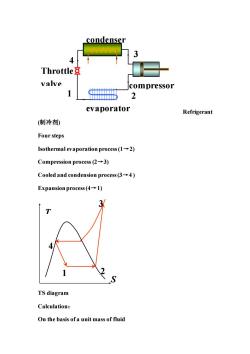
condenser 3 Throttle valve compressor 2 evaporator Refrigerant (制冷剂 Four steps Isothermal evaporation process(1-2) Compression process(2-3) Cooled and condension process(3-4) Expansion process(4-1) 3 TS diagram Calculation: On the basis ofa unit mass of fluid
Refrigerant (制冷剂) Four steps Isothermal evaporation process (1→2) Compression process (2→3) Cooled and condension process (3→4 ) Expansion process (4→1) TS diagram Calculation: On the basis of a unit mass of fluid condenser 4 1 2 3 compressor Throttle valve evaporator S T 4 1 2 3
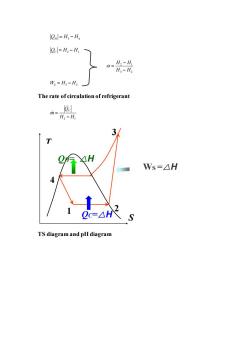
n=H3-H Qc=H,-H 0=4-月 H3-H2 Ws Hs-H2 The rate of circulation of refrigerant HH Ws=△H 4 0 c=△H TS diagram and pH diagram
Q H H H = − 3 4 Q H H C = − 2 1 2 1 3 2 H H H H − = − W H H S = − 3 2 The rate of circulation of refrigerant 2 1 QC m H H = − TS diagram and pH diagram S T 4 1 2 3 QH= △H QC=△H WS =△H
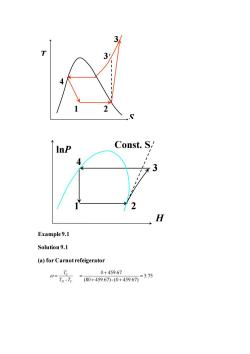
InP Const.S/ 4 2 H Example9.1 Solution9.1 (a)for Carnotrefeigerator 0+459.67 07n元=80+459.671-0+459675.75
Example 9.1 Solution 9.1 (a) for Carnot refeigerator - C H C T T T = 0 459.67 5.75 (80 459.67) - (0 459.67) + = = + + S T 4 1 2 3 3′ lnP H 2 3 4 1 Const. S

condenser 4 Throttle8 valve compressor 1 t 2 evaporator 换算273*9/5-32 (b)for the vapor-compression cycle H3-H2 H1 H2 H3? T2 saturated vapor S. T4 saturated liquid 用→W=-H,→ 亿→3'))isentropy S=S, W=W/n→H=-用+H2 (4→1)isenthalpy,=H Refrigeration capacity(制冷能力) Qe h
换算 273*9/5-32 (b) for the vapor-compression cycle 2 1 3 2 H H H H − = − H1 H2 H3? T2 saturated vapor H S2 2 T4 saturated liquid H P P 4 4 3 = H3 W H H SR 3 2 = − (2 → 3 ′ ) isentropy 3 2 S S = / W W s SR = H W H 3 2 = − + s (4 → 1) isenthalpy H H 1 4 = Refrigeration capacity (制冷能力) / Q kJ h C 2 1 QC m H H = − condenser 4 1 2 3 compressor Throttle valve evaporator

Thinking(9-1) 1.Coefficient of performance for Carnot refrigerator is the function of temperature only 01n-1 2.在相同温度区间工作的制冷循环,以逆卡诺循环的制冷系数为最 大。 3.制冷循环中,高温下放热量大于低温下吸热量。 4+4 -+g4=Q+W0=Q+用.-l+w=0g.+W= 2 Thinking(9-1) 4.The definition of coefficient of performance is that the heat absorbed at a lower temperature is divided by work that compressor needs heat absorbed at the lower temperatre net work
Thinking(9-1) 1. Coefficient of performance for Carnot refrigerator is the function of temperature only C H C T T T = − 2. 在相同温度区间工作的制冷循环,以逆卡诺循环的制冷系数为最 大。 3. 制冷循环中,高温下放热量大于低温下吸热量。 2 2 s u H g z Q W + + = + 0 = + Q Ws 0 Q Q W C − + = H s C Q W Q + =s H Thinking(9-1) 4. The definition of coefficient of performance is that the heat absorbed at a lower temperature is divided by work that compressor needs heat absorbed at the lower temperature C net wor Q k W = = S T 4 1 2 3 3 ′

9.3 The Choice of Refrigerant 9.3.1 Theimportant characteristics of Refrigerant Toxicity flammability cost corrosion property vapor pressure in related to temperature 大气压力下沸点低,不仅可获得较低的制冷温度,且在一定制冷温度 下,蒸汽压力高于大气压,防止空气进入 常温下冷凝压力应尽量低,以降低冷凝器的耐压与密封要求 汽化潜热大,减少制冷剂循环量,缩小压缩机尺寸;具有较高临界温 度与较低凝固温度,使大部分放热过程在两相区进行:具有化学稳定 性,不易燃,不分解,无腐蚀。 9.3.2 Refrigerants (1)Conventional refrigerants: NH3 CH3CI C02 C3H8 (2)Chloro Fluoro Carbons CFC's CFCl3(R-11)CF2Cl2(R-12) (3)Hydro Chloro Fluoro Carbons HCFC's: CHCIF(R-22) CHCI2CF3(R-123) (4)(Hydro)Fluoro Carbons HFC's
9.3 The Choice of Refrigerant 9.3.1 Theimportant characteristics of Refrigerant Toxicity flammability cost corrosion property vapor pressure in related to temperature 大气压力下沸点低,不仅可获得较低的制冷温度,且在一定制冷温度 下,蒸汽压力高于大气压,防止空气进入; 常温下冷凝压力应尽量低,以降低冷凝器的耐压与密封要求 汽化潜热大,减少制冷剂循环量,缩小压缩机尺寸;具有较高临界温 度与较低凝固温度,使大部分放热过程在两相区进行;具有化学稳定 性,不易燃,不分解,无腐蚀。 9.3.2 Refrigerants (1) Conventional refrigerants: NH3 CH3Cl CO2 C3H8 (2) Chloro Fluoro Carbons CFC’s CFCl3 (R-11) CF2Cl2 (R-12) (3) Hydro Chloro Fluoro Carbons HCFC’s : CHCl2F (R-22) CHCl2CF3 (R-123) (4) (Hydro) Fluoro Carbons HFC’s
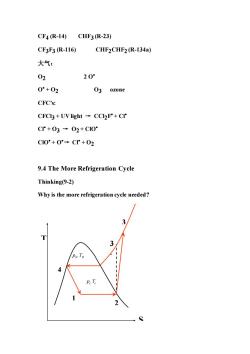
CF4(R-14)CHF3R-23) CF3F3R-116) CHF2CHF2(R-134a) 大气: 02 20 0°+02 O3 ozone CFC's: CFCI3 +UV light CCIF+CI* C+03→02+C10 C10°+0°→Cr+02 9.4 The More Refrigeration Cycle Thinking(9-2) Why is the more refrigeration cycle needed? 3 p
CF4 (R-14) CHF3 (R-23) CF3F3 (R-116) CHF2CHF2 (R-134a) 大气: O2 2 O• O• + O2 O3 ozone CFC’s: CFCl3 + UV light → CCl2F• + Cl• Cl• + O3 → O2 + ClO• ClO• + O•→ Cl• + O2 9.4 The More Refrigeration Cycle Thinking(9-2) Why is the more refrigeration cycle needed? S T 4 1 2 3 3 H H ’ p T c c p T
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《化工热力学》课程授课教案(讲义)Chapter 8 Production of Power from Heat.doc
- 《化工热力学》课程授课教案(讲义)Chapter 7 Application of Thermodynamics to Flow Processes.doc
- 《化工热力学》课程授课教案(讲义)Chapter 6 Thermodynamic properties of fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 5 The Second Law of Thermodynamics.doc
- 《化工热力学》课程授课教案(讲义)Chapter 3 Volumetric Properties of Pure Fluids.doc
- 《化工热力学》课程授课教案(讲义)Chapter 2 The First Law and Other Basic Concepts.doc
- 《化工热力学》课程教学大纲 Thermodynamics of chemical engineering(新疆大学:黄雪莉).doc
- 海南大学:《精细化学品与工艺学》课程授课教案(讲义,共十章,负责人:刘钟馨、韩秀萍).docx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)食品添加剂 Food additives.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(醇酸树脂涂料、丙烯酸树脂涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)表面活性剂 surfactants.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)合成材料助剂.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)胶黏剂.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)绪论.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(环氧树脂涂料、聚氨酯树脂涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料(涂料的概念、作用和组成).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)涂料分类(涂料的分类和命名、溶剂型涂料、水性涂料).pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)有机染料和颜料.pptx
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)化妆品.ppt
- 海南大学:《精细化学品与工艺学》课程教学课件(PPT讲稿)香料.ppt
- 《化工热力学》课程授课教案(讲义)Chapter 11 Solution thermodynamics - Theory.doc
- 《化工热力学》课程授课教案(讲义)Chapter 12 Solution Thermodynamics:Application.doc
- 《化工热力学》课程授课教案(讲义)Chapter 13 Chemical-Reaction Equilibria.doc
- 《化工热力学》课程教学资源(作业习题)第1章 绪言(无答案).doc
- 《化工热力学》课程教学资源(作业习题)第2章 流体的P-V-T关系(含答案).doc
- 《化工热力学》课程教学资源(作业习题)第4章 非均相封闭体系热力学(选择题带答案).doc
- 《化工热力学》课程教学资源(作业习题)第5章 非均相体系热力学性质计算(无答案).doc
- 《化工热力学》课程教学资源(作业习题)第8章 化学反应平衡(含答案).doc
- 《化工热力学》课程教学课件(英文讲稿)纯流体性质 Volumetric Properties of Pure Fluids.pdf
- 《化工热力学》课程教学课件(英文讲稿)流体热力学性质 Thermodynamic properties of fluids.pdf
- 《化工热力学》课程教学课件(英文讲稿)热动力 Production of Power from Heat.pdf
- 《化工热力学》课程教学课件(英文讲稿)溶液热力学原理 Solution thermodynamics:Theory.pdf
- 《化工热力学》课程教学课件(英文讲稿)热功转换及可用能.pdf
- 《化工热力学》课程教学课件(PPT讲稿)第一章 绪论 Chemical Engineering thermodynamics.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第二章 流体的p-V-T关系.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第五章 化工过程的能量分析.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第七章 相平衡.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第三章 流体的热力学性质.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第六章 蒸汽动力循环与制冷循环.ppt
- 《化工热力学》课程教学课件(PPT讲稿)第十章 化学反应平衡.ppt
