北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes

1.What would be the name of the following? 5-cyclopentyl-1-methyleyclononane cis-1-cyclopentyl-5-methylcyclodecane cis-5-methyl-1-cyclopentylcyclododecane trans-cyclopentyl-5-methylcyclodecane (5-methylcyclodecyl)cyclopentane Ans: A)cis-1,2-dimethylcyclohexane trans-1,3-dimethylcyclohexane cis-1.4-dimethyleyclohexane Ans: 3.Terpenes can be considered to be built up from what units? c) D) E) Ans:
Page 1 1. What would be the name of the following? CH3 A) 5-cyclopentyl-1-methylcyclononane B) cis-1-cyclopentyl-5-methylcyclodecane C) cis-5-methyl-1-cyclopentylcyclododecane D) trans-cyclopentyl-5-methylcyclodecane E) (5-methylcyclodecyl)cyclopentane Ans: B 2. Which of the following isomers would you expect to have the lowest heat of combustion? (i.e., be most stable) A) cis-1,2-dimethylcyclohexane B) trans-1,3-dimethylcyclohexane C) cis-1,4-dimethylcyclohexane D) cis-1,3-dimethylcyclohexane E) All should have the same heat of combustion. Ans: D 3. Terpenes can be considered to be built up from what units? A) B) C) D) E) Ans: E

4.Which of the following compounds has the highest heat of combustion per CH cyclohexane B) 5.What is the correct IUPAC name for the following molecule: A)1-ethyl-2-methylhexane D)1-ethyl-2-methylcyclohexane 2-ethyl-1-methylcycloheptane Ans: Dethyl-5-methyleyeloe E)1-methyloctane CH(CH3) 3) 9 aa H.C D) CH(CH3). A CH(CH3) Ans:E Page2
Page 2 4. Which of the following compounds has the highest heat of combustion per CH2 group? A) cyclopropane D) cyclohexane B) cyclobutane E) All have equal ΔHcombustion. C) cyclopentane Ans: A 5. What is the correct IUPAC name for the following molecule: A) 1-ethyl-2-methylhexane D) 1-ethyl-2-methylcyclohexane B) 2-ethyl-1-methylcycloheptane E) 1-methyloctane C) 4-ethyl-5-methylcyclohexane Ans: D 6. Which would be the most stable conformation of trans-1-methyl-3-isopropylcyclohexane? A) CH(CH3) H 2 H3C H B) CH(CH3) CH 2 3 H H C) CH(CH3)2 H H3C H D) CH(CH3)2 H CH3 H E) CH H 3 H CH(CH3)2 Ans: E

7.Which one of the following cyclic alkanes has the greatest tendency to have a planar cyclohexan none of the above are planar Ans:A 8.What would be the proper name of the following: (C)3C 才H cis-1-tert-butyl-4-methylcyclohexane trans-1-tert-butyl-4-methylcyclohexane axial.equatorial-1-tert-butyl-4-methylcyclohexane cis-1-isopropyl-4-methylcyclohexane opopy-meyleyclohe 9.The most stable conformation of cis 1.3-dimethylcyclohexane has how many hydrogen atoms in axial positions? A)4 B)5 C)6 D)8 E)none of the above Ans:C 10.Which of the following stituted cyclohexanes could exist in a conformation is-4-dimethylcyclohexane trans-1,3-dimethylcyclohexane cis-1,2-dimethylcyclohexane E) All or none can have both groups equatorial s:A Page 3
Page 3 7. Which one of the following cyclic alkanes has the greatest tendency to have a planar ring? A) cyclopropane D) cyclohexane B) cyclobutane E) none of the above are planar C) cyclopentane Ans: A 8. What would be the proper name of the following: CH3 H H (CH3)3C A) cis-1-tert-butyl-4-methylcyclohexane B) trans-1-tert-butyl-4-methylcyclohexane C) axial,equatorial-1-tert-butyl-4-methylcyclohexane D) cis-1-isopropyl-4-methylcyclohexane E) trans-1-isopropyl-4-methylcyclohexane Ans: A 9. The most stable conformation of cis 1,3-dimethylcyclohexane has how many hydrogen atoms in axial positions? A) 4 B) 5 C) 6 D) 8 E) none of the above Ans: C 10. Which of the following disubstituted cyclohexanes could exist in a conformation that has both groups equatorial? A) cis-1,3-dimethylcyclohexane B) cis-1,4-dimethylcyclohexane C) trans-1,3-dimethylcyclohexane D) cis-1,2-dimethylcyclohexane E) All or none can have both groups equatorial. Ans: A
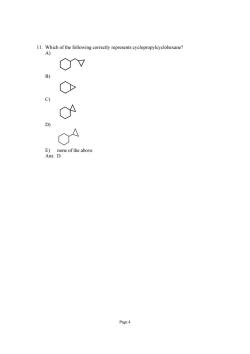
11. 0v O C) n Done of the above Page
Page 4 11. Which of the following correctly represents cyclopropylcyclohexane? A) B) C) D) E) none of the above Ans: D

hichth olngng systems belongs to the class of compounds calld strod Page 5
Page 5 12. Which of the following ring systems belongs to the class of compounds called steroids? A) B) C) D) E) Ans: B
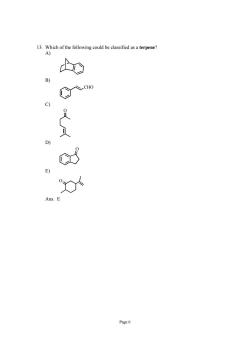
⊙ Page6
Page 6 13. Which of the following could be classified as a terpene? A) B) CHO C) O D) O E) O Ans: E
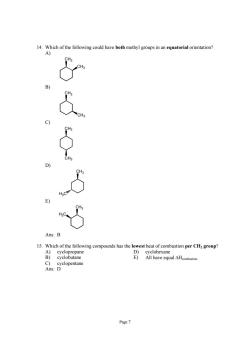
14.Which of the following could have both methyl groups in an equatorial orientation? Ans:B 15.Which of the following compounds has the lowest heat of combustion per CH2 group? A)cyclopropane cyclobutane
Page 7 14. Which of the following could have both methyl groups in an equatorial orientation? A) CH3 CH3 B) CH3 CH3 C) CH3 CH3 D) H3C CH3 E) CH3 H3C Ans: B 15. Which of the following compounds has the lowest heat of combustion per CH2 group? A) cyclopropane D) cyclohexane B) cyclobutane E) All have equal ΔHcombustion. C) cyclopentane Ans: D

Which,if either,of the two isomers of the compound shown below would be more (trans) (cis) Cis is more stable Neither is stable. rans is more stable There is no way to predict this oth are equally stable 17.Although five-and six-membered rings are generally the most stable,why is cyclopentane less stable than cyclohexane? A) The angles in cyclopentane deviate significantly from the tetrahedral angle. Five-membered rings have trans annular interactions. red rings have eclipsing hydrogen o s closer to 109 ns 18.Which of the following correctly shows the Newman projection along a C-C bond in cyclohexane?(the squiggles indicate where the rest of the ring is attached) CH2 H CH A H, CH-Z E AB)B C)C D)DE Page 8
Page 8 16. Which, if either, of the two isomers of the compound shown below would be more stable? (trans) (cis) A) Cis is more stable. D) Neither is stable. B) Trans is more stable. E) There is no way to predict this. C) Both are equally stable. Ans: B 17. Although five- and six-membered rings are generally the most stable, why is cyclopentane less stable than cyclohexane? A) The angles in cyclopentane deviate significantly from the tetrahedral angle. B) Five-membered rings have trans annular interactions. C) Five-membered rings have eclipsing hydrogens. D) Planar cyclohexane has bond angles closer to 109°. E) Larger rings are always more stable than smaller rings. Ans: C 18. Which of the following correctly shows the Newman projection along a C-C bond in cyclohexane? (the squiggles indicate where the rest of the ring is attached) H CH H 2 H CH H 2 H H H H CH2 CH2 H H CH2 H CH2 H H H H H CH CH2 2 H H H CH2 CH2 H A BC D E A) A B) B C) C D) D E) E Ans: D

19.Which of the following cyclic alkanes can be ring-opened under hydrogenation conditions H, eyelic hydrocarbon linear hydrocarbon Pd A)cyclopropane D)cyclohexane cyclobutane E)more than one of these C cyclopentane Ans: 20.Which of the following statements about conformations of methyleyelohexane is true? CHs AN CH A)The energy barrier to interconvert these is too high to be achieved at room temperature. 9 The two forms are in equilibrium and are present in equal amounts at room temperature. The two forms are not in equilibrium but are present in equal amounts at room D) emperatu ms are in equilibrium but are not present in equal amounts at room atur E)The two forms are not in equilibrium and are not present in equal amounts at room temperature. Ans:D 21.Cyclohexanes exhibit a higher than their straight-chain analogs.(Choose the D) All of these R e are co of the se are correc Ans:D .Which conformation of cyclohexane exprinc thmon A) Chair B)Planar C)Boat E)All of these are Page 9
Page 9 19. Which of the following cyclic alkanes can be ring-opened under hydrogenation conditions? cyclic hydrocarbon H2 Pd linear hydrocarbon A) cyclopropane D) cyclohexane B) cyclobutane E) more than one of these C) cyclopentane Ans: E 20. Which of the following statements about conformations of methylcyclohexane is true? CH3 CH3 A) The energy barrier to interconvert these is too high to be achieved at room temperature. B) The two forms are in equilibrium and are present in equal amounts at room temperature. C) The two forms are not in equilibrium but are present in equal amounts at room temperature. D) The two forms are in equilibrium but are not present in equal amounts at room temperature. E) The two forms are not in equilibrium and are not present in equal amounts at room temperature. Ans: D 21. Cyclohexanes exhibit a higher_______than their straight-chain analogs. (Choose the correct answer) A) boiling point D) All of these are correct. B) melting point E) Two of these are correct. C) density Ans: D 22. Which conformation of cyclohexane experiences the most transannular strain? A) Chair B) Planar C) Boat D) Twist boat E) All of these are stable. Ans: C

23.Which of the following is o in its most stable conformation? h C D E)All of these are in their most stable conformation. Ans:A 24.What isthe poteial energy changeo fromstbomaion? 45 Kcal/mol Ans:C 25.Which of the following structures represent cis-1,4-dimethyleyclohexane? H CHa A)I&ⅡB)I&llC)Ⅱ&lⅢlD)all of the above E)none of the above Ans:B Page 10
Page 10 23. Which of the following is not in its most stable conformation? A) Cl CH3 B) I CH2CH3 C) H3C OCH3 D) HOOC Br E) All of these are in their most stable conformation. Ans: A 24. What is the potential energy change to convert from a twist-boat to boat conformation? A) –14 Kcal/mol D) 14 Kcal/mol B) 0 Kcal/mol E) 45 Kcal/mol C) 1.4 Kcal/mol Ans: C 25. Which of the following structures represent cis-1,4-dimethylcyclohexane? H CH3 CH3 H I CH3 H CH3 H II CH3 H H H3C III A) I & II B) I & III C) II & III D) all of the above E) none of the above Ans: B
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试题.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷.pdf
- 《有机化学》课程教学资源(文献资料)[3,3]σ迁移反应过渡态立体化学过程的新观点.pdf
- 《有机化学》课程教学资源(文献资料)芳香过渡态理论及其在协同反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)芳香性概念的新发展.pdf
- 《有机化学》课程教学资源(文献资料)对映体识别_不对称合成中的新概念.pdf
- 《有机化学》课程教学资源(文献资料)多环体系的芳香性.pdf
- 《有机化学》课程教学资源(文献资料)多烯化合物的Cope重排反应——[5,5]σ迁移、串联[3,3]σ迁移与自由基重排机理.pdf
- 《有机化学》课程教学资源(文献资料)E2反应中的反式共平面构象.pdf
- 《有机化学》课程教学资源(文献资料)各类手性质子源及其在去消旋化反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)分子内氢键对化合物性质的影响.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 22 Chemistry of Benzene Substituents:Alkylbenzenes, Phenols, and Benzenamines.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 23 Ester Enolates and the Claisen Condensation:Synthesis of b-Dicarbonyl Compounds; Acyl Anion Equivalents.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 24 Carbohydrates:Polyfunctional Compounds in Nature.pdf
