北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis

To which side,if any,would the following equilibrium lie? c H; CH-O Na"H2O 之 CH-OH NaOH A)to the left B)to the right Btsonegom this reaction cannot occur at all c Ans ul to the righ and le 2.What would be the name of the following compound? HO 2-sec-butyl-1-butanol 2-(sec-pentyl)-1-butanol thyl-3-methyl-I-hexano C
Page 1 1. To which side, if any, would the following equilibrium lie? H3C CH H3C OH H3C CH H3C O- Na+ + H2O + NaOH A) to the left D) there is no way to tell B) to the right E) this reaction cannot occur at all C) equally to the right and left Ans: B 2. What would be the name of the following compound? HO A) 3-hydroxymethyl-4-methylheptane D) 2-ethyl-3-propyl-1-butanol B) 2-sec-butyl-1-butanol E) 2-(sec-pentyl)-1-butanol C) 2-ethyl-3-methyl-1-hexanol Ans: C
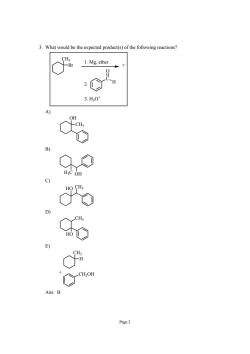
3.What would be the expected product(s)of the following reactions? o% 1.Mg.ether 3.H30 Page2
Page 2 3. What would be the expected product(s) of the following reactions? CH3 Br C O H ? 1. Mg, ether 2. 3. H3O+ A) CH3 OH B) H3C OH C) HO CH3 D) CH3 HO E) CH3 H CH2OH + Ans: B
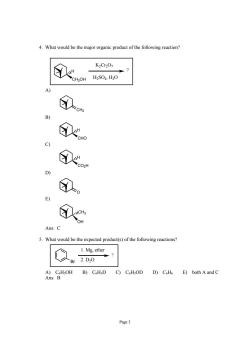
4.What would be the major organic product of the following reaction? 8 K2Cr207 A ①n ①o 9 ①o D) 。 E) ① Ans:C 5.What would be the expected product(s)ofthe following reactions? 1.Mg.ether 人Br2.D0 Ans B)CH.D D)CoH6 E)both A and C Page3
Page 3 4. What would be the major organic product of the following reaction? CH2OH H ? K2Cr2O7 H2SO4, H2O A) CH2 B) CHO H C) CO2H H D) O E) CH3 OH Ans: C 5. What would be the expected product(s) of the following reactions? Br ? 1. Mg, ether 2. D2O A) C6H5OH B) C6H5D C) C6H5OD D) C6H6 E) both A and C Ans: B
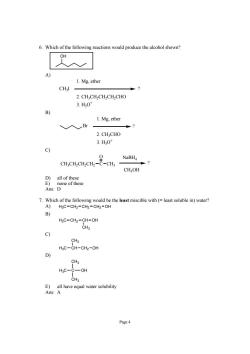
6.Which of the following reactions would produce the alcohol shown? 1.Mg,ether 2.CH:CHCHCH CHO 3.H0 B) 1.Mg,ether 、入Br 2.CH;CHO 3.H,0 9 NaBH p7 CH;OH mre re Ans:D 7.Which of the following would be the least miscible with(=least soluble in)water? A)H3C-CH2-CH2-CH2-OH B) C--o C) HsC-CH-CH2-OH D) CH3 HgC-C-OH Page4
Page 4 6. Which of the following reactions would produce the alcohol shown? OH A) CH3I 1. Mg, ether 2. CH3CH2CH2CH2CHO 3. H3O+ ? B) Br ? 1. Mg, ether 2. CH3CHO 3. H3O+ C) CH3CH2CH2CH2 C O CH3 NaBH4 CH3OH ? D) all of these E) none of these Ans: D 7. Which of the following would be the least miscible with (= least soluble in) water? A) H3C CH2 CH2 CH2 OH B) CH3 H3C CH2 CH OH C) CH3 H3C CH CH2 OH D) CH3 CH3 H3C C OH E) all have equal water solubility Ans: A
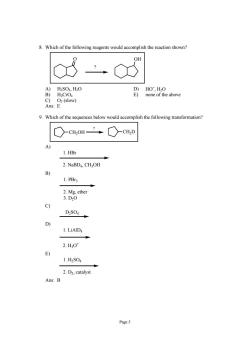
8.Which of the following reagents would accomplish the reaction shown? 8 OH A)H2S04,H0 B)H.CrO c Oz(slow) Ans:E 9.Which of the sequences below would accomplish the following transformation? ○cH,OH”○cH,D 1.HBr 2.NaBD4,CHOH B 1.PB3 2.Mg,ether 3.D0 C) D2S04 D) 2.H0t 1.H2S04 2.D2,catalyst Ans:B
Page 5 8. Which of the following reagents would accomplish the reaction shown? O OH ? A) H2SO4, H2O D) HO− , H2O B) H2CrO4 E) none of the above C) O2 (slow) Ans: E 9. Which of the sequences below would accomplish the following transformation? CH2OH CH2D ? A) 1. HBr 2. NaBD4, CH3OH B) 1. PBr3 2. Mg, ether 3. D2O C) D2SO4 D) 1. LiAlD4 2. H3O+ E) 1. H2SO4 2. D2, catalyst Ans: B

10.Which of the following five-carbon alcohols would you expect to be the most soluble in ater? A CH3-CH2-CH2-CH2-CH2OH OH CH3-CH2-CH2-CH-CH3 c) OH CHa-CH2-CH-CH2-CH3 D) CH3-CH2-CH-CH2OH CH3 E) CH3- -CH2OH CHa Ans:E 11.What reagent(s)would accomplish the following transformation? CD2OH A) Pyridinium chlorochromate B)LiAIDa then H:O' Ans:A 12.Which statement about the preparation of Grignard reagents is otrue? RX+Mg→RMgX Methyl.primary,secondary,tertiary and aryl halides may be used D)Rearrangement may accompany Grignard formation. E)The halogen may be chloride,bromide,or iodide. Ans:D Page6
Page 6 10. Which of the following five-carbon alcohols would you expect to be the most soluble in water? A) CH3 CH2 CH2 CH2 CH2OH B) CH3 CH2 CH2 CH CH3 OH C) CH3 CH2 CH CH2 CH3 OH D) CH3 CH2 CH CH2OH CH3 E) CH3 CH3 C CH2OH CH3 Ans: E 11. What reagent(s) would accomplish the following transformation? CD2OH C O ? D A) Pyridinium chlorochromate D) Potassium dichromate B) LiAlD4 then H3O+ E) H2SO4 C) H2CrO4 Ans: A 12. Which statement about the preparation of Grignard reagents is not true? RX + Mg RMgX A) The reaction must be done in an ether solvent. B) The reagent should be protected from air. C) Methyl, primary, secondary, tertiary and aryl halides may be used. D) Rearrangement may accompany Grignard formation. E) The halogen may be chloride, bromide, or iodide. Ans: D
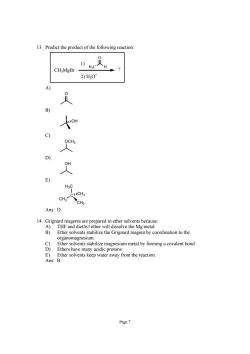
3.Predict the product of the following reaction CHMgBr 2)H0 3 C ) CH Ans:D Ether solvents stabilize the Grignard reagent by coordination to the organomagnesium. 8 Ether solvents stabilize magnesium metal by forming a covalent bond Ethers have many acidic protons. E) Ether solvents keep water away from the reaction. Ans Page7
Page 7 13. Predict the product of the following reaction: CH3MgBr 1) 2) H3O+ ? H3C H O A) O B) OH C) OCH3 D) OH E) CH3 C H3C CH3 CH3 Ans: D 14. Grignard reagents are prepared in ether solvents because: A) THF and diethyl ether will dissolve the Mg metal. B) Ether solvents stabilize the Grignard reagent by coordination to the organomagnesium. C) Ether solvents stabilize magnesium metal by forming a covalent bond. D) Ethers have many acidic protons. E) Ether solvents keep water away from the reaction. Ans: B
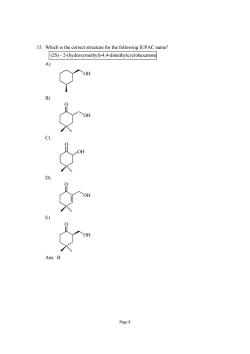
15.Which is the correct structure for the following IUPAC name? (2S)-2-(hydroxymethyl)-4,4-dimethylcyclohexanone OH Page8
Page 8 15. Which is the correct structure for the following IUPAC name? (2S) - 2-(hydroxymethyl)-4,4-dimethylcyclohexanone A) OH B) O OH C) O OH D) O OH E) O OH Ans: B
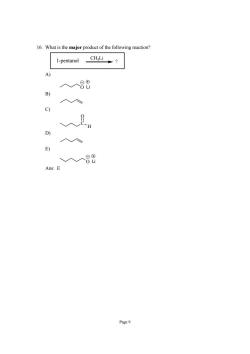
16.What is the major product of the following reaction 1-pentanol cHi→? 入人88 B c) D) 入入 E) 人人8 Ans:E Page9
Page 9 16. What is the major product of the following reaction? 1-pentanol CH3Li ? A) O Li B) C) C H O D) E) O Li Ans: E
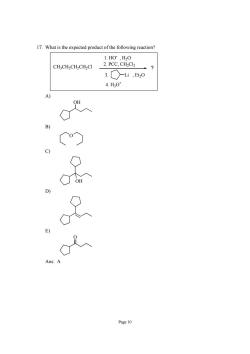
17.What is the expected product of the following reaction? 1.H0,H,0 CH;CH2CH2CH2CI 2.PCC.CHCI 3.○-i,,0 4.H0 Page 10
Page 10 17. What is the expected product of the following reaction? Li CH3CH2CH2CH2Cl 1. HO- , H2O 2. PCC, CH2Cl2 3. , Et2O 4. H3O+ ? A) OH B) O C) OH D) E) O Ans: A
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试题.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷.pdf
- 《有机化学》课程教学资源(文献资料)[3,3]σ迁移反应过渡态立体化学过程的新观点.pdf
- 《有机化学》课程教学资源(文献资料)芳香过渡态理论及其在协同反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)芳香性概念的新发展.pdf
- 《有机化学》课程教学资源(文献资料)对映体识别_不对称合成中的新概念.pdf
- 《有机化学》课程教学资源(文献资料)多环体系的芳香性.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 22 Chemistry of Benzene Substituents:Alkylbenzenes, Phenols, and Benzenamines.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 23 Ester Enolates and the Claisen Condensation:Synthesis of b-Dicarbonyl Compounds; Acyl Anion Equivalents.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 24 Carbohydrates:Polyfunctional Compounds in Nature.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 25 Heterocycles:Heteroatoms in Cyclic Organic Compounds.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 26 Amino Acids, Peptides, Proteins, and Nucleic Acids:Nitrogen-Containing Polymers in Nature.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
