北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules

1.The correct name of the following molecule would be A)2-ethyl-3.3-dimethylheptane B)6-ethyl-5,5-dimethylheptane cane C) 3,4,4-trimethyloctane Ans:C 2.What is the name given to the Newman p butane conformation show CH A)anti B)gauche C)staggered D)eclipsed E)skewed Ans:B 3.At room temperature,the various conformations of butane B not inte E)There is no way to determine if interconversion occurs Ans:D Page
Page 1 1. The correct name of the following molecule would be: A) 2-ethyl-3,3-dimethylheptane D) 2-butyl-3-methylpentane B) 6-ethyl-5,5-dimethylheptane E) 2-sec-butyl-2-methylhexane C) 3,4,4-trimethyloctane Ans: C 2. What is the name given to the Newman projection of the butane conformation shown here? H H CH3 CH3 H H A) anti B) gauche C) staggered D) eclipsed E) skewed Ans: B 3. At room temperature, the various conformations of butane A) do not interconvert with only the anti form present. B) do not interconvert, but all forms are present. C) interconvert slowly. D) interconvert rapidly. E) There is no way to determine if interconversion occurs. Ans: D
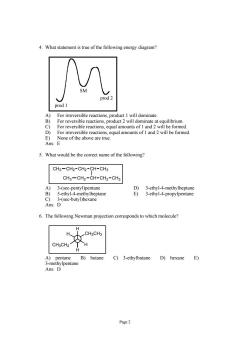
4.What statement is true of the following energy diagram? SM prod 1 For irreversible reaction dominate at for re eversible reactions.equal amounts of l and 2 will be formed D) For irreversible reactions,equal amounts of 1 and 2 will be formed. E) None of the above are true. Ans:E 5.What would be the correct name of the following CH3-CH2-CH2-CH-CH3 CH3-CH2-CH-CH2-CH 3-(se pentane 6.The following Newman projection corresponds to which molecule? H CH2CH3 CH3CH2 A)pentane B)butane C)3-ethylbutane D)hexane E) Page2
Page 2 4. What statement is true of the following energy diagram? prod 1 prod 2 SM A) For irreversible reactions, product 1 will dominate. B) For reversible reactions, product 2 will dominate at equilibrium. C) For reversible reactions, equal amounts of 1 and 2 will be formed. D) For irreversible reactions, equal amounts of 1 and 2 will be formed. E) None of the above are true. Ans: E 5. What would be the correct name of the following? CH2 CH2 CH CH3 CH3 CH2 CH CH2 CH3 CH3 A) 3-(sec-pentyl)pentane D) 3-ethyl-4-methylheptane B) 5-ethyl-4-methylheptane E) 3-ethyl-4-propylpentane C) 3-(sec-butyl)hexane Ans: D 6. The following Newman projection corresponds to which molecule? H CH3CH2 H H CH2CH3 H A) pentane B) butane C) 3-ethylbutane D) hexane E) 3-methylpentane Ans: D
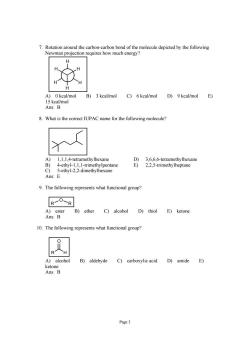
7.Rotation around the carbon- arbon bond of the molecule depicted by the following Newman projection requires how much energy A)0kcal/mol B)3 kcal/mol C)6 keal/mol D)9 kcal/mol E) 8.What is the correct IUPAC name for the following molecule? A) 1,1,1,4-tetramethylhexane D)3.6.6.6-tetramethylhexane 4-ethyl-1,1,1-trimethylpentane E)2,2,5-trimethylheptane -ethyl-2,2-dimethylhexane Ans: 9.The following represents what functional group? R7O-R A)ester B)ether C)alcohol D)thiol E)ketone Ans:B 10.The following represents what functional group? A)alcohol B)aldehyde C)carboxylic acid D)amide E) Page3
Page 3 7. Rotation around the carbon-carbon bond of the molecule depicted by the following Newman projection requires how much energy? H H H H H H A) 0 kcal/mol B) 3 kcal/mol C) 6 kcal/mol D) 9 kcal/mol E) 15 kcal/mol Ans: B 8. What is the correct IUPAC name for the following molecule? A) 1,1,1,4-tetramethylhexane D) 3,6,6,6-tetramethylhexane B) 4-ethyl-1,1,1-trimethylpentane E) 2,2,5-trimethylheptane C) 5-ethyl-2,2-dimethylhexane Ans: E 9. The following represents what functional group? R R O A) ester B) ether C) alcohol D) thiol E) ketone Ans: B 10. The following represents what functional group? R H O A) alcohol B) aldehyde C) carboxylic acid D) amide E) ketone Ans: B
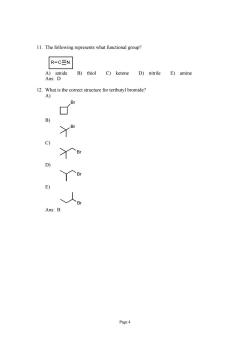
11.The following represents what functional group? R-CEN B)thiol C)ketone D)nitrile E)amine 12.What is the correct structure for tertbutyl bromide? A) B) 丫e 人 Page4
Page 4 11. The following represents what functional group? R C N A) amide B) thiol C) ketone D) nitrile E) amine Ans: D 12. What is the correct structure for tertbutyl bromide? A) Br B) Br C) Br D) Br E) Br Ans: B

13.The following Newman projection represents which molecule? CH3 CH2CH3 CH2CH2CH3 Page5
Page 5 13. The following Newman projection represents which molecule? H H3CH2CH2C CH3 CH2CH3 CH3 CH2CH2CH3 A) B) CH3 CH3 C) D) E) Ans: A
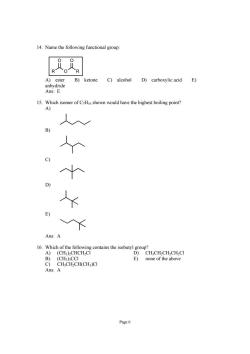
14.Name the following functional group: R R ketone C)alcohol D)carboxylic acid E) 15.Which isomer of CHis shown would have the highest boiling point? A) Ans:A D)CH.CH.CH.CH.CI (CH3)CCI E)none of the above C)CH;CH2CH(CH)CI Ans:A Page6
Page 6 14. Name the following functional group: ROR O O A) ester B) ketone C) alcohol D) carboxylic acid E) anhydride Ans: E 15. Which isomer of C7H16 shown would have the highest boiling point? A) B) C) D) E) Ans: A 16. Which of the following contains the isobutyl group? A) (CH3)2CHCH2Cl D) CH3CH2CH2CH2Cl B) (CH3)3CCl E) none of the above C) CH3CH2CH(CH3)Cl Ans: A

17.Which of the follo H.CH.CH.CH.CI CHCH-CH(CH3)CI AnsC 18.How would the following molecule be properly named? CH3 CH3-CH CH3CH2-CH HC-CH2CH2CH3 CH2 CHa 3-ethyl-2-methyl-4- D)3-isopropyl-4-propylhexane B) opyiepne 3.4-diethyl-2-methylheptane E)none of the above 19. p) 24.4-trimethylheptane 3-tert-butyl-2.2-dimethylbutane none of the above C) 2.2,3,4,4-pentamethylpentane Ans:C 20.What is the smalles C)pentane E)all are liquids 21.All steroids are derivatives of the ring system shown.How many tertiary hydrogens are in this ring system? 〔 EeB剧24D)5目6 Page7
Page 7 17. Which of the following contains the sec-butyl group? A) (CH3)2CHCH2Cl D) CH3CH2CH2CH2Cl B) (CH3)3CCl E) None of the above C) CH3CH2CH(CH3)Cl Ans: C 18. How would the following molecule be properly named? CH3 CH CH CH3 HC CH2 CH3 CH3CH2 CH2CH2CH3 A) 3-ethyl-2-methyl-4-propylhexane D) 3-isopropyl-4-propylhexane B) 3,4-diethyl-2-methylheptane E) none of the above C) 4-ethyl-3-isopropylheptane Ans: B 19. The IUPAC name for [(CH3)3C]2CHCH3 is: A) 1,1-di-tert-butylethane D) 2,4,4-trimethylheptane B) 3-tert-butyl-2,2-dimethylbutane E) none of the above C) 2,2,3,4,4-pentamethylpentane Ans: C 20. What is the smallest alkane that is liquid at room temperature? A) propane B) butane C) pentane D) hexane E) all are liquids Ans: C 21. All steroids are derivatives of the ring system shown. How many tertiary hydrogens are in this ring system? A) none B) 2 C) 4 D) 5 E) 6 Ans: E
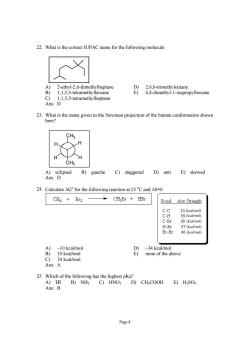
22.What is the correct IUPAC name for the following molecule: )2-ethyl-2.6-dimethylheptane D)2.6,6-trimethyloctane B) 1,1,5,5-tetramethylhexane E)4,4-dimethyl-1-isopropylhexane Ans: -etramethytheptane 23.What is the name given to the Newman projection of the butane conformation shown here? CHa A)eclipsed B) gauche C)staggered D)anti E)skewed Ans:D 24.Calculate AG°for the following reaction at25℃and△S-0 CH4 Br2 CHBr HBr Bond Ave Strengt地 C-C 83 (kcal/mol 99 (kcal/mol Br-Br 46(keal/mol) -34 kcal/r none of the above Ans:A 25.Which of the following has the highest pKa? A)HI B)NH3 C)HNO3 D)CHsCOOH E)H2SO4 Ans:B Page 8
Page 8 22. What is the correct IUPAC name for the following molecule: A) 2-ethyl-2,6-dimethylheptane D) 2,6,6-trimethyloctane B) 1,1,5,5-tetramethylhexane E) 4,4-dimethyl-1-isopropylhexane C) 1,1,5,5-tetramethylheptane Ans: D 23. What is the name given to the Newman projection of the butane conformation shown here? CH3 H H CH3 H H A) eclipsed B) gauche C) staggered D) anti E) skewed Ans: D 24. Calculate ΔGo for the following reaction at 25 o C and ΔS=0 A) –10 kcal/mol D) –34 kcal/mol B) 10 kcal/mol E) none of the above C) 34 kcal/mol Ans: A 25. Which of the following has the highest pKa? A) HI B) NH3 C) HNO3 D) CH3COOH E) H2SO4 Ans: B
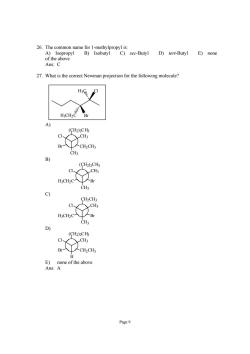
Ans:C 27.What is the correct Newman projection for the following molecule? H3CH2C (CH2)2CHz C、 CH3 (CH2)2CH H:CH2C- Br CH3 c) CH2CH3 CH3 H;CH2C CH b) (CH2)2CHs C、 CH3 -CH2CH E)none of the above Ans:A Page9
Page 9 26. The common name for 1-methylpropyl is: A) Isopropyl B) Isobutyl C) sec-Butyl D) tert-Butyl E) none of the above Ans: C 27. What is the correct Newman projection for the following molecule? H3C Cl H3CH2C Br A) CH3 Cl CH3 (CH2)2CH3 Br CH2CH3 B) CH3 Cl CH3 (CH2)2CH3 H3CH2C Br C) CH3 Cl CH3 CH2CH3 H3CH2C Br D) H Cl CH3 (CH2)2CH3 Br CH2CH3 E) none of the above Ans: A
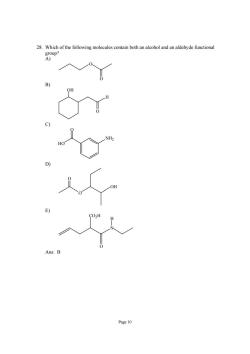
28.Which of the following molecules contain both an alcohol and an aldehyde functional oup? Page 10
Page 10 28. Which of the following molecules contain both an alcohol and an aldehyde functional group? A) O O B) H O OH C) HO NH2 O D) O OH O E) N CO2H O H Ans: B
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试题.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷.pdf
- 《有机化学》课程教学资源(文献资料)[3,3]σ迁移反应过渡态立体化学过程的新观点.pdf
- 《有机化学》课程教学资源(文献资料)芳香过渡态理论及其在协同反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)芳香性概念的新发展.pdf
- 《有机化学》课程教学资源(文献资料)对映体识别_不对称合成中的新概念.pdf
- 《有机化学》课程教学资源(文献资料)多环体系的芳香性.pdf
- 《有机化学》课程教学资源(文献资料)多烯化合物的Cope重排反应——[5,5]σ迁移、串联[3,3]σ迁移与自由基重排机理.pdf
- 《有机化学》课程教学资源(文献资料)E2反应中的反式共平面构象.pdf
- 《有机化学》课程教学资源(文献资料)各类手性质子源及其在去消旋化反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)分子内氢键对化合物性质的影响.pdf
- 《有机化学》课程教学资源(文献资料)共轭效应和芳香性本质的争论和它们的历史发展.pdf
- 《有机化学》课程教学资源(文献资料)含手性碳原子的[3,3]σ迁移反应的立体化学过程.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 22 Chemistry of Benzene Substituents:Alkylbenzenes, Phenols, and Benzenamines.pdf
