北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 01 Structure and Bonding in Organic Molecules

1sceokarmhoimnaec时rokopoundnoaga A)C1oHis B)C1oH20 C)C1oHi6 D)CnH4 E)CuHis Ans:C 2.Of those indicated,which would be the shortest carbon-carbon bond in a-selinene? A)A B)B C)C D)D E)E Ans:B What would be the ideal value for the indicated bond angle? C)104° D)180°E)109
Page 1 1. What is the molecular formula of limonene, the major volatile compound in orange peel oil? limonene A) C10H18 B) C10H20 C) C10H16 D) C11H14 E) C11H18 Ans: C 2. Of those indicated, which would be the shortest carbon-carbon bond in α-selinene? E D C B A A) A B) B C) C D) D E) E Ans: B 3. What would be the ideal value for the indicated bond angle? C H Br A) 120° B) 90° C) 104° D) 180° E) 109° Ans: E
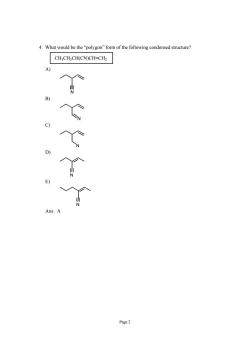
What would be the"polygon"form of the following condensed structure CHCH2CH(CN)CH=CHz Page2
Page 2 4. What would be the “polygon” form of the following condensed structure? CH3CH2CH(CN)CH=CH2 A) N B) N C) N D) N E) N Ans: A
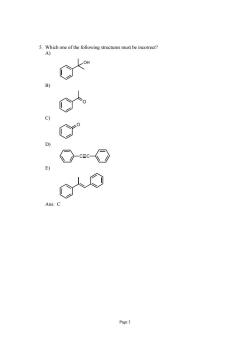
.Whch of h following structures must nore 0以0 Page
Page 3 5. Which one of the following structures must be incorrect? A) OH B) O C) O D) C C E) Ans: C
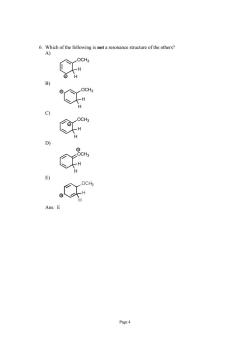
not a resonance structure of the others? Page 4
Page 4 6. Which of the following is not a resonance structure of the others? A) OCH3 H H + B) OCH3 H H + C) OCH3 H H + D) OCH3 H H + E) Ans: E
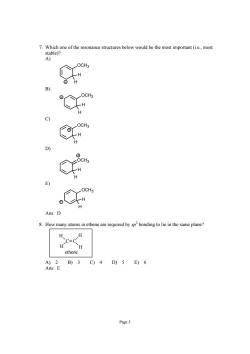
Which one of the resonance structures below would be the most important(ie..most How many atoms in ethene are required by spbonding to lie in the same plane? c=c ethene 0234D)56 Page 5
Page 5 7. Which one of the resonance structures below would be the most important (i.e., most stable)? A) OCH3 H H + B) OCH3 H H + C) OCH3 H H + D) OCH3 H H + E) OCH3 H H + Ans: D 8. How many atoms in ethene are required by sp 2 bonding to lie in the same plane? C C H H H H ethene A) 2 B) 3 C) 4 D) 5 E) 6 Ans: E
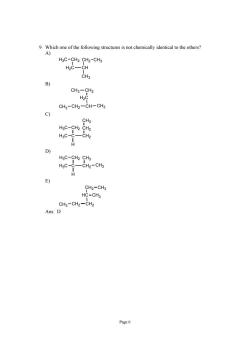
9.Which one of the following structures is not chemically identical to the others? HgC-CH2 CH2-CH3 H2C- -CH CHa CH3-CH2 H2C CH3-CH2-CH-CH3 CH3 HgC-CH2 CH2 H3C-CCH2 D) H3C-CH2 CHs H3C-0 -CH2-CH3 夕 CH2-CH3 HC-CH3 CH3-CH2-CH2 Ans:D Page6
Page 6 9. Which one of the following structures is not chemically identical to the others? A) H3C CH2 H2C CH CH2 CH3 CH3 B) CH3 CH2 H2C CH3 CH2 CH CH3 C) H3C H3C CH2 C CH2 CH2 CH3 H D) H3C H3C CH2 C CH2 CH3 CH3 H E) CH2 CH3 HC CH3 CH2 CH2 CH3 Ans: D
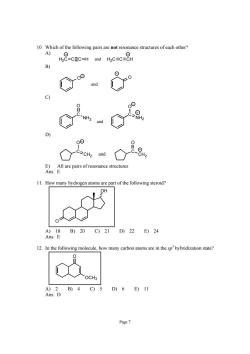
10.Which of the following pairs are not resonance structures of each other? A) H-CEC-H d MC=C=CH B) a and合 11.How many hydrogen atoms are part of the following steroid? OH A)18B)20C)21D)22E)24 Ans:E 2.In the following molecule,how many carbon atoms are in the sphybridization state 〔文 OCH A)2B)4C)5D)6E)11 Ans:D Page7
Page 7 10. Which of the following pairs are not resonance structures of each other? A) H2CCCH H and 2C C CH - - B) O O - - and C) C NH2 O C NH2 O- + and D) C CH2 O C CH2 O - - and E) All are pairs of resonance structures Ans: E 11. How many hydrogen atoms are part of the following steroid? O OH A) 18 B) 20 C) 21 D) 22 E) 24 Ans: E 12. In the following molecule, how many carbon atoms are in the sp 3 hybridization state? OCH3 O A) 2 B) 4 C) 5 D) 6 E) 11 Ans: D
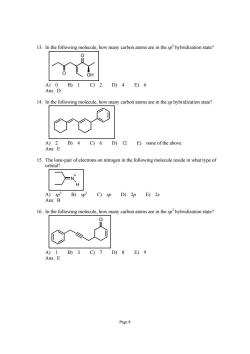
13.In the following molecule,how many carbon atoms are in the sp2hybridization state? A)0B)1C)2D)4E)6 Ans:D 14.In the following molecule,how many carbon atoms are in the sp hybridization state? B)4C)6 D)12 E)none of the above 15.The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? C)sp D)2p E)2s 16.In the following molecule how many carbon atoms are in the sphybridization state A)1B)3C)7D)8E)9 Ans:E Page 8
Page 8 13. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O O OH A) 0 B) 1 C) 2 D) 4 E) 6 Ans: D 14. In the following molecule, how many carbon atoms are in the sp hybridization state? A) 2 B) 4 C) 6 D) 12 E) none of the above Ans: E 15. The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? H N A) sp 3 B) sp 2 C) sp D) 2p E) 2s Ans: B 16. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O A) 1 B) 3 C) 7 D) 8 E) 9 Ans: E

17.The boxed item most likely represents what? none of the above Ans 18.The following molecule contains how many carbon atoms in the sp hybridization state? Ais:A B)3 C)8D)13E)16 19.The nitrogen A) methyineCHNconshor B)one c) molecule two D)three Ans:B 20.A positive charge on oxygen generally occurs when: oxygen has too many electrons. oxygen has too few electrons. onding pairs 21.The carbon atom in CH2Cl2 has what hybridization? C)sp'D)sp*E)they are not hybridized 22.The molecular formula for piperitone is piperitone A)CHi6O B)C1oHisO C)C.HisO D)C10H4O E)C10H16O Ans:E Page9
Page 9 17. The boxed item most likely represents what? A) s orbital D) could be any of A–C B) sp 3 orbital E) none of the above C) p orbital Ans: B 18. The following molecule contains how many carbon atoms in the sp hybridization state? C O A) 1 B) 3 C) 8 D) 13 E) 16 Ans: A 19. The nitrogen of trimethylamine [(CH3)3N] contains how many lone pairs of electrons? A) none B) one C) two D) three E) there is no nitrogen in this molecule Ans: B 20. A positive charge on oxygen generally occurs when: A) oxygen has too many electrons. B) oxygen has too few electrons. C) oxygen is sharing one of its non-bonding electron pairs. D) oxygen has too many non-bonding electron pairs. E) oxygen is borrowing electrons from another atom. Ans: C 21. The carbon atom in CH2Cl2 has what hybridization? A) sp B) sp 2 C) sp 3 D) sp 4 E) they are not hybridized Ans: C 22. The molecular formula for piperitone is O piperitone A) C9H16O B) C10H18O C) C9H18O D) C10H14O E) C10H16O Ans: E

23.Which structure is different from the others? CHCHCICHICH C-CH3 D 3 CH5-CH CI-CH-CH3 及alre enteal geosmin? (-)-geosmi A)C1H200B)C12H220 C)CuH21O D)C12H20O E)C12H21O Ans:B carbo-carbn bondsndicaed od you expettobthonges A)A B)B C)C D)D E)E Ans:D Page 10
Page 10 23. Which structure is different from the others? A) Cl B) CH3CHClCH(CH3)2 C) CH3 C C Cl CH3 H3C H H D) CH3 CH CH CH3 Cl CH3 E) all are identical Ans: E 24. A fairly common algal metabolite is the compound (-)-geosmin, which imparts a musty odor to water even at concentrations in the ppb range. What is the molecular formula of geosmin? (-)-geosmin CH3 HO CH3 A) C11H20O B) C12H22O C) C11H21O D) C12H20O E) C12H21O Ans: B 25. Which of the carbon-carbon bonds indicated would you expect to be the longest in stilbene? A B C D E A) A B) B C) C D) D E) E Ans: D
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试题.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷.pdf
- 《有机化学》课程教学资源(文献资料)[3,3]σ迁移反应过渡态立体化学过程的新观点.pdf
- 《有机化学》课程教学资源(文献资料)芳香过渡态理论及其在协同反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)芳香性概念的新发展.pdf
- 《有机化学》课程教学资源(文献资料)对映体识别_不对称合成中的新概念.pdf
- 《有机化学》课程教学资源(文献资料)多环体系的芳香性.pdf
- 《有机化学》课程教学资源(文献资料)多烯化合物的Cope重排反应——[5,5]σ迁移、串联[3,3]σ迁移与自由基重排机理.pdf
- 《有机化学》课程教学资源(文献资料)E2反应中的反式共平面构象.pdf
- 《有机化学》课程教学资源(文献资料)各类手性质子源及其在去消旋化反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)分子内氢键对化合物性质的影响.pdf
- 《有机化学》课程教学资源(文献资料)共轭效应和芳香性本质的争论和它们的历史发展.pdf
- 《有机化学》课程教学资源(文献资料)含手性碳原子的[3,3]σ迁移反应的立体化学过程.pdf
- 《有机化学》课程教学资源(文献资料)关于芳香性的概念.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
