北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity
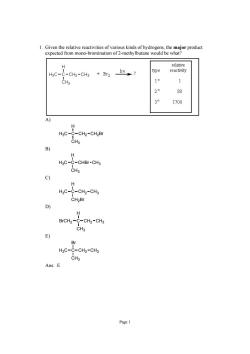
1.Given the relative reactivities of various kinds of hydrogens.the major product expected from mono-bromination of 2-methylbutane would be what H3C- -CH2-CH3 +Br2 hv? 1° 1 80 1700 -CH2-CH2Br CHa B) H HgC-C-CHBr-CH3 CH3 9 H HgC-C-CH2-CH3 CH2Br H BrCH2-C-CH2-CH3 E) Br HaC-C-CHz-CHj CH3 Ans:E
Page 1 1. Given the relative reactivities of various kinds of hydrogens, the major product expected from mono-bromination of 2-methylbutane would be what? A) H3C C CH3 H CH2 CH2Br B) H3C C CH3 H CHBr CH3 C) H3C C CH2Br H CH2 CH3 D) BrCH2 C CH3 H CH2 CH3 E) H3C C CH3 Br CH2 CH3 Ans: E

2.Which of the following is a propagation step in the free-radical bromination of B) Bra hy 2Br. C)CH3+Br2- CH;Br Br. D)CHy+CH: CH3CH3 E)Br?+Br? Br2 Ans:C 3.Which of the reactions below would you expect to have the largest positive A? A) CH:Br B) Br2 hy 2 Br. c) CH3 Br2- CH;Br Br. D) CH3CH3 →CH,CH3 E)Br?+Br? Br2 Ans:B 4.What reactant is needed to complete and balance the following reaction? +302spark 3CO +3H0 A)C3Hs B)C2H6 C)C:Ha D)C:H6 E)CH2 Ans:D 5hnenscsbdwnnomgeyawcomtpgti。 A)CH3 I?CHjl B) 2hv+2. C)CH+I- →CH,I+I D)CH+一 →CH?+HⅢ E)I?+I?- 12 Ans:D Page2
Page 2 2. Which of the following is a propagation step in the free-radical bromination of methane? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: C 3. Which of the reactions below would you expect to have the largest positive Δ? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: B 4. What reactant is needed to complete and balance the following reaction? ? + 3 O2 3 CO + 3 H2O spark A) C3H8 B) C2H6 C) C3H4 D) C3H6 E) C6H12 Ans: D 5. Which one of the reactions below will not proceed and accounts for why iodine added to a free-radical chlorination or bromination will greatly slow or even stop the reaction? A) CH3• + I? CH3I B) hν I2 2 I• C) CH3 CH I + I• 3• + I2 D) CH4 + I• CH3? + HI E) I? + I? I2 Ans: D

6.Which of the following is not true of free-radical halogenation reactions? n chlorine in these reactions. s more s minations are faster emperatures to proceed These reactions are irreversible. Ans:D 7.Which of the following reaction types are typical of alkanes? on more than one of these is correct titution 8.Alkanes are noted for their(choose one) A)high reactivity with acids. D)lack of reactivity B)toxicity. E)water-solubility odor. Page3
Page 3 6. Which of the following is not true of free-radical halogenation reactions? A) Fluorine is more reactive than chlorine in these reactions. B) Bromine is more selective than chlorine. C) The reactions require either light or high temperatures to proceed. D) Brominations are faster than chlorinations. E) These reactions are irreversible. Ans: D 7. Which of the following reaction types are typical of alkanes? A) addition D) reduction B) elimination E) more than one of these is correct C) radical substitution Ans: C 8. Alkanes are noted for their (choose one) A) high reactivity with acids. D) lack of reactivity. B) toxicity. E) water-solubility. C) intense odor. Ans: D

9.What is the major product of the following reaction? C ) Page4
Page 4 9. What is the major product of the following reaction? + Br2 ? light A) Br B) Br C) Br D) Br Br E) no reaction occurs Ans: A
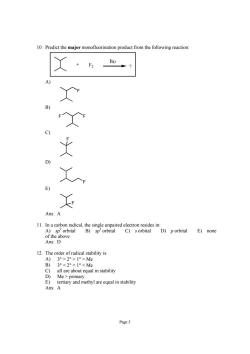
10.Predict the major monofluorination product from the following reaction: C) D) Ans:A 11.In a carbon radical,the single unpaired electron resides in: A)sp'orbital B)sp'orbital C)s orbital D)porbital E)none 12.The order of radical stability is: 3°>20>1>Me 3°<20<1°<Me Mepi all are about equal in stability E)tertiary and methyl are equal in stability Ans:A Page 5
Page 5 10. Predict the major monofluorination product from the following reaction: + ? hυ F2 A) F B) F F C) F D) F E) F Ans: A 11. In a carbon radical, the single unpaired electron resides in: A) sp 2 orbital B) sp 3 orbital C) s orbital D) p orbital E) none of the above Ans: D 12. The order of radical stability is: A) 3° > 2° > 1° > Me B) 3° primary E) tertiary and methyl are equal in stability Ans: A

The transition state les the reactants The transition state resembles the products E)both Band C Ans:C ronjation s most useful for stabilizing which of the follow 15.In a carbon radical,the carbon possesses how many valence electrons? A)4 B)5 C)6 D)7 E)8 Ans:D 6ee of the following would you choose to accomplish the reaction showr CO C0 (X=halogen) A)F2.light B)Cl2.light C)Br2.light D)I2.light E)none of the above Ans:C Page6
Page 6 13. Which of the following is true for an early transition state? A) The starting materials resemble the products. B) The reaction proceeds very slowly. C) The transition state resembles the reactants. D) The transition state resembles the products. E) both B and C Ans: C 14. Hyperconjugation is most useful for stabilizing which of the following? A) neopentyl radical D) ethyl radical B) tert-butyl radical E) methyl radical C) isopropyl radical Ans: B 15. In a carbon radical, the carbon possesses how many valence electrons? A) 4 B) 5 C) 6 D) 7 E) 8 Ans: D 16. Which, if any, of the following would you choose to accomplish the reaction shown below in the best yield? X (X = halogen) A) F2, light B) Cl2, light C) Br2, light D) I2, light E) none of the above Ans: C
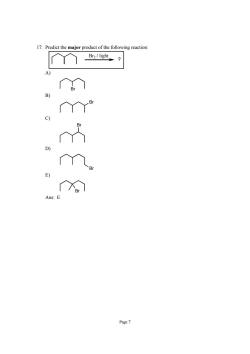
17.Predict the major product of the following reaction: Br2/light C) Page7
Page 7 17. Predict the major product of the following reaction: Br2 / light ? A) Br B) Br C) Br D) Br E) Br Ans: E

18.Predict the major product of the following reaction 3 E)no reaction Ans:E 19 ueoavctity because o-selective 8 a late transition state is involved. an early transition state is involved. E)fluorine is too unreactive. Ans:D 20.Which of the carbon-carbon bonds indicated would be the weakest? A)A B)B C)C D)D E)E Ans:E Page8
Page 8 18. Predict the major product of the following reaction: I2 / light ? A) I B) I C) I D) E) no reaction Ans: E 19. Free-radical fluorination demonstrates little regio-selectivity because A) fluorine is too small to have steric hinderance. B) free-radical reactions are never regio-selective. C) a late transition state is involved. D) an early transition state is involved. E) fluorine is too unreactive. Ans: D 20. Which of the carbon-carbon bonds indicated would be the weakest? H3C A B C E D A) A B) B C) C D) D E) E Ans: E

21.Which alkane below would you expect to have the lowest heat of combustion? A) B) C) lwould be the same. 22.Which of the following would you not expect to be formed in even small amounts during the free-radical chlorination of methane? B) CH;CH3 Page9
Page 9 21. Which alkane below would you expect to have the lowest heat of combustion? A) B) C) D) E) All would be the same. Ans: C 22. Which of the following would you not expect to be formed in even small amounts during the free-radical chlorination of methane? A) CH2Cl2 B) CH3CH3 C) CHCl3 D) CCl4 E) Some of all of these would be formed. Ans: E

23.Given the relative reactivities of var ious kinds of hydrogen ected from mono-chlorination of 2-methylbutane would be whpr relative H HgC-C-CH2-CH3 Cl2 hv? type reactivity 3° A) H H3C-C-CH2-CH2Cl CH3 H H3C-C-CHCl-CH3 CH3 9 H HaC-C-CHz-CHa CH2CI D) 9 CHg-C-CH2-CH3 CH3 E)There is no way to predict this. Ans:B Page 10
Page 10 23. Given the relative reactivities of various kinds of hydrogens, the major product expected from mono-chlorination of 2-methylbutane would be what? A) H3C C CH3 H CH2 CH2Cl B) H3C C CH3 H CHCl CH3 C) H3C C CH2Cl H CH2 CH3 D) CH3 C CH3 Cl CH2 CH3 E) There is no way to predict this. Ans: B
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试题.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机B期中试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期末试卷.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷及其答案.pdf
- 北京化工大学:《有机化学》课程教学资源(模拟试卷)有机A期中试卷.pdf
- 《有机化学》课程教学资源(文献资料)[3,3]σ迁移反应过渡态立体化学过程的新观点.pdf
- 《有机化学》课程教学资源(文献资料)芳香过渡态理论及其在协同反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)芳香性概念的新发展.pdf
- 《有机化学》课程教学资源(文献资料)对映体识别_不对称合成中的新概念.pdf
- 《有机化学》课程教学资源(文献资料)多环体系的芳香性.pdf
- 《有机化学》课程教学资源(文献资料)多烯化合物的Cope重排反应——[5,5]σ迁移、串联[3,3]σ迁移与自由基重排机理.pdf
- 《有机化学》课程教学资源(文献资料)E2反应中的反式共平面构象.pdf
- 《有机化学》课程教学资源(文献资料)各类手性质子源及其在去消旋化反应中的应用.pdf
- 《有机化学》课程教学资源(文献资料)分子内氢键对化合物性质的影响.pdf
- 《有机化学》课程教学资源(文献资料)共轭效应和芳香性本质的争论和它们的历史发展.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 22 Chemistry of Benzene Substituents:Alkylbenzenes, Phenols, and Benzenamines.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 23 Ester Enolates and the Claisen Condensation:Synthesis of b-Dicarbonyl Compounds; Acyl Anion Equivalents.pdf
