北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 22 Chemistry of Benzene Substituents:Alkylbenzenes, Phenols, and Benzenamines

1.Which ion would be most stabilized by resonance? A)】 g 9 ④ D C CH-CHa E)all are equally stabilized Ans:B 之c6ee8 o0odck时r structure c) E)all are equally important Ans:D
Page 1 1. Which ion would be most stabilized by resonance? A) CH2CH2 B) CHCH3 C) CH2CH3 D) CH2CH3 E) all are equally stabilized Ans: B 2. Which of the following resonance structures of the phenoxide anion would be the major contributor to the real structure? A) O B) O C) O D) O E) all are equally important Ans: D
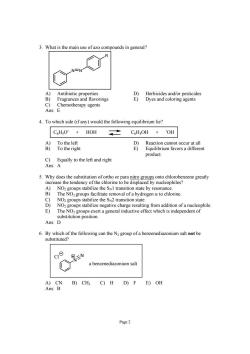
3.What is the main use of azo compounds in general? A) Antibiotic properties D)Herbicides and/or pesticides B) Fragrances and flavorings E)Dyes and coloring agents Chemotherapy agents Ans: 4.To which side(if any)would the following equilibrium lie? C6HO+HOH CHsOH +OH C)Equally to the left and right Ans:A 5.Why does the substitution of ortho or para nito groups onto chlorobenzene greatly the e to be displ d by nucleophiles n state by resc nanc sin a hy rogen a to chlorine NO. negative charge resulting from addition of a nucleophile E) The NOz groups exert a general inductive effect which is independent of substitution position. Ans:D 6.By which of the following can the N group of a benzenediazonium salt not be substituted? c N a benzenediazonium salt A)CN B)CH3 C)H D)F E)OH Ans:B Page2
Page 2 3. What is the main use of azo compounds in general? N N R A) Antibiotic properties D) Herbicides and/or pesticides B) Fragrances and flavorings E) Dyes and coloring agents C) Chemotherapy agents Ans: E 4. To which side (if any) would the following equilibrium lie? C6H5O- + HOH C6H5OH + - OH A) To the left D) Reaction cannot occur at all B) To the right E) Equilibrium favors a different product. C) Equally to the left and right Ans: A 5. Why does the substitution of ortho or para nitro groups onto chlorobenzene greatly increase the tendency of the chlorine to be displaced by nucleophiles? A) NO2 groups stabilize the SN1 transition state by resonance. B) The NO2 groups facilitate removal of a hydrogen α to chlorine. C) NO2 groups stabilize the SN2 transition state. D) NO2 groups stabilize negative charge resulting from addition of a nucleophile. E) The NO2 groups exert a general inductive effect which is independent of substitution position. Ans: D 6. By which of the following can the N2 group of a benzenediazonium salt not be substituted? N N a benzenediazonium salt Cl A) CN B) CH3 C) H D) F E) OH Ans: B

7.What material is produced easily by means of the Kolbe reaction?(sodium phenoxide heat/pressure,then HO) Succinic acid Ans:E 8.What product would result from the following reaction? heat D OHC E) None ofthese Ans: 9.What happens when a secondary amine is treated with nitrous acid? A)A diazonium salt is formed. It immediately loses nitrogen to form a carbocation. A simple acid-base reaction occurs to give an alkyl ammonium nitrite salt. troso amine is formed D dealkylation occurs to give a primary amine Page 3
Page 3 7. What material is produced easily by means of the Kolbe reaction? (sodium phenoxide + CO2 + heat/pressure, then H3O+ ) A) Phenol D) Cinnamic acid B) Benzaldehyde E) Salicylic acid C) Succinic acid Ans: E 8. What product would result from the following reaction? O ? heat A) O D) OHC B) O E) None of these C) O H Ans: C 9. What happens when a secondary amine is treated with nitrous acid? A) A diazonium salt is formed. B) It immediately loses nitrogen to form a carbocation. C) A simple acid-base reaction occurs to give an alkyl ammonium nitrite salt. D) An N-nitroso amine is formed. E) A dealkylation occurs to give a primary amine. Ans: D
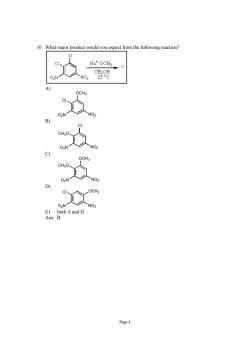
0 What major product would you expect from the following reaction? Na*OCH 、c8 Page 4
Page 4 10. What major product would you expect from the following reaction? A) Cl OCH3 O2N NO2 B) CH3O Cl O2N NO2 C) CH3O OCH3 O2N NO2 D) Cl OCH3 O2N NO2 E) both A and D Ans: B
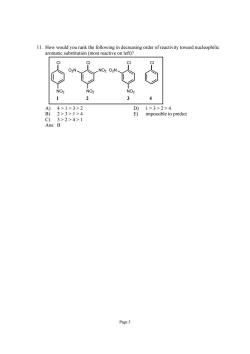
11.How would you rank the following in decreasing order of reactivity toward nucleophilic aromatic substitution (most reactive on left)? 4>1>3>2 2>3>1>4 impossible to predict 3>2>4>1 B Page 5
Page 5 11. How would you rank the following in decreasing order of reactivity toward nucleophilic aromatic substitution (most reactive on left)? Cl Cl Cl NO2 O2N NO2 Cl NO2 O2N NO2 1 2 34 A) 4 > 1 > 3 > 2 D) 1 > 3 > 2 > 4 B) 2 > 3 > 1 > 4 E) impossible to predict C) 3 > 2 > 4 > 1 Ans: B

What would be the result of the following Page6
Page 6 12. What would be the result of the following? O ? heat A) O B) O C) HO D) O E) no reaction Ans: C

3 What would be the expected product of the following reaction o.c Page 7
Page 7 13. What would be the expected product of the following reaction? O O + ? A) O O B) C) O O D) O O E) O Ans: D

14.What product would you expect from the following reaction? KMnO4 Page8
Page 8 14. What product would you expect from the following reaction? CH2 CH2 KMnO4 H2O, NaOH heat ? A) O O B) O O- Na+ 2 C) CH3 2 D) CH2OH 2 E) CH3 HOCH2 + Ans: B
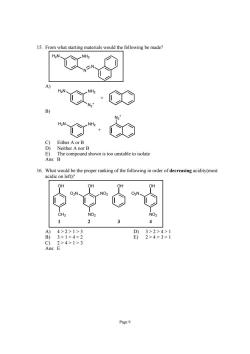
15.From what starting materials would the following be made? X·Ce E The compound shown is too unstable to isolate Ans:B 16.What would be the proper ranking of the following in order of decreasing acidity(most acidic on left)? A)4>2>1>3 B)3>1>4>2 Page9
Page 9 15. From what starting materials would the following be made? N N H2N NH2 A) N2 + H2N NH2 + B) H2N NH2 N2 + + C) Either A or B D) Neither A nor B E) The compound shown is too unstable to isolate Ans: B 16. What would be the proper ranking of the following in order of decreasing acidity(most acidic on left)? OH OH OH CH3 O2N NO2 OH NO2 O2N NO2 1 2 3 4 A) 4 > 2 > 1 > 3 D) 3 > 2 > 4 > 1 B) 3 > 1 > 4 > 2 E) 2 > 4 > 3 > 1 C) 2 > 4 > 1 > 3 Ans: E
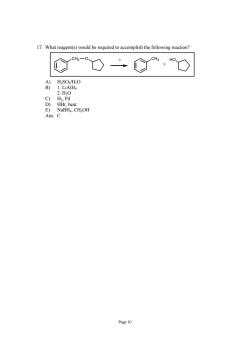
17.What reagent(s)would be required to accomplish the following reaction? C%O二C%,O A)H2SO4/H2O B)1.LiAlH 2.H0 Page 10
Page 10 17. What reagent(s) would be required to accomplish the following reaction? CH2 O CH3 HO + ? A) H2SO4/H2O B) 1. LiAlH4 2. H2O C) H2, Pd D) HBr, heat E) NaBH4, CH3OH Ans: C
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 21 Amines and Their Derivatives:Functional Groups Containing Nitrogen.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 20 Carboxylic Acid Derivatives.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 19 Carboxylic Acids.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 18 Enols, Enolates, and the Aldol Condensation:a, b-Unsaturated Aldehydes and Ketones.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 17 Aldehydes and Ketones:The Carbonyl Group.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 23 Ester Enolates and the Claisen Condensation:Synthesis of b-Dicarbonyl Compounds; Acyl Anion Equivalents.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 24 Carbohydrates:Polyfunctional Compounds in Nature.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 25 Heterocycles:Heteroatoms in Cyclic Organic Compounds.pdf
- 北京化工大学:《有机化学》课程教学资源(章节测验,含答案)Chapter 26 Amino Acids, Peptides, Proteins, and Nucleic Acids:Nitrogen-Containing Polymers in Nature.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 01 Structure and Bonding in Organic Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 02 Structure and Reactivity:Acids and Bases, Polar and Nonpolar Molecules.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 03 Reactions of Alkanes:Bond-Dissociation Energies, Radical Halogenation, and Relative Reactivity.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 04 Cycloalkanes.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 05 Stereoisomers.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 06 Properties and Reactions of Haloalkanes - Bimolecular Nucleophilic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 07 Further Reactions of Haloalkanes:Unimolecular Substitution and Pathways of Elimination.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 08 Hydroxy Functional Group:Alcohols:Properties, Preparation, and Strategy of Synthesis.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 09 Further Reactions of Alcohols and the Chemistry of Ethers.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 10 Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 11 Alkenes; Infrared Spectroscopy and Mass Spectrometry.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 12 Reactions of Alkenes.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 13 Alkynes:The Carbon–Carbon Triple Bond.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 14 Delocalized Pi Systems:Investigation by Ultraviolet and Visible Spectroscopy.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 15 Benzene and Aromaticity:Electrophilic Aromatic Substitution.pdf
- 北京化工大学:《有机化学》课程教学资源(习题与答案)Chapter 16 Electrophilic Attack on Derivatives of Benzene:Substituents Control Regioselectivity.pdf
