上海交通大学:《材料热力学》教学资源_2018 lecture 7 equilibrium

Contents of Today S.J.T.U. Phase Transformation and Applications Review previous Condition of equilibrium Phase equilibrium Clapeyron equation in vapor equilibria Variation of vapor pressure with particle size Second-order transition SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Contents of Today Review previous Condition of equilibrium Phase equilibrium Clapeyron equation in vapor equilibria Variation of vapor pressure with particle size Second-order transition
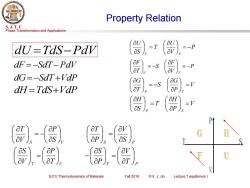
Property Relation S.J.T.U. Phase Transformation and Applications aU dU-Tds-Pdv =T =-P dF=-SdT-Pav =-S 8) dG=-SdT+Vdp =-S =V dH=TaS+Vap -T =V 二 as ap) H as aP as ap SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Property Relation S S V P V T = − S S P V P T = T T V P V S = T T P V P S = − dU =TdS−PdV dF = −SdT−PdV dG= −SdT+VdP dH =TdS+VdP P V U T S U V S = − = P V F S T F V T = − = − V P G S T G P T = = − V P H T S H P S = =
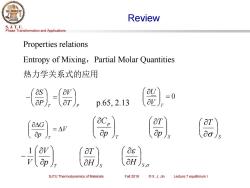
Review S.J.T.U. Phase Transformation and Applications Properties relations Entropy of Mixing,Partial Molar Quantities 热力学关系式的应用 -) p.65..13 a△G =△V ap ap (av 0s OH aH S,o SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Review Properties relations Entropy of Mixing,Partial Molar Quantities 热力学关系式的应用 T T P V P S = − = 0 T V U p.65, 2.13 V p G T = T p p C S p T S T T p V V − 1 H S T H S,

Quiz Q3 Answer S.J.T.U. Phase Transformation and Applications 15.进行下述过程时,系统的△U、△H、△S和△G何者为零? (1)非理想气体的卡诺循环; (2)隔离系统中的任意过程: (3)在100℃,01325Pa下1mol水蒸发成水蒸气; (4)绝热可逆过程。 15题:1)内能变化为0,焓变为0,熵变为0,自由能变化为0。 2):内能变化为0, 焓变不一定为0,熵变不一定为0,自由能变化不 一定为0。 3)自由能变化为0。错,主要认为相变过程中内能为0,焓变也为0。 4)只有熵变为0。错误很多。 SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Quiz Q3 Answer 15题:1)内能变化为0,焓变为0,熵变为0,自由能变化为0。 2):内能变化为0,焓变不一定为0,熵变不一定为0,自由能变化不 一定为0。 3)自由能变化为0。错,主要认为相变过程中内能为0,焓变也为0。 4)只有熵变为0。错误很多
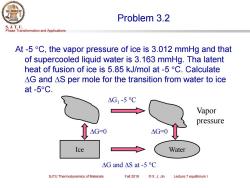
Problem 3.2 S.J.T.U. Phase Transformation and Applications At-5 C,the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg.Tha latent heat of fusion of ice is 5.85 kJ/mol at -5C.Calculate AG and AS per mole for the transition from water to ice at-5°C. △G1-5C Vapor pressure △G=0 △G=-0 Ice Water △Gand△Sat-5C SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Problem 3.2 At -5 C, the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg. Tha latent heat of fusion of ice is 5.85 kJ/mol at -5 C. Calculate G and S per mole for the transition from water to ice at -5C. Ice Water G and S at -5 C G1 -5 C G=0 G=0 Vapor pressure

Index of nomenclature S.J.T.U. Phase Transformation and Applications Equilibrium:平衡 Phase equilibrium:相平衡 First order transitions:一级相变 Second-order transition:二级相变 SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Index of nomenclature Equilibrium: 平衡 Phase equilibrium: 相平衡 First order transitions: 一级相变 Second-order transition: 二级相变

Introduction to equilibrium S.J.T.U. Phase Transformation and Applications The concept of equilibrium is fundamental Stable,unchanging with time and certain properties of the system are uniform throughout The system may not be homogeneous in form Co-existence of ice and water Phase A portion of matter that is uniform throughout,not only in chemical composition,but also in physical state - The usefulness of many metallic,polymeric,and ceramic systems depends on the presence,at equilibrium,of various different phases in the material 组织 SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Introduction to equilibrium • The concept of equilibrium is fundamental • Stable, unchanging with time and certain properties of the system are uniform throughout • The system may not be homogeneous in form – Co-existence of ice and water • Phase – A portion of matter that is uniform throughout, not only in chemical composition, but also in physical state – The usefulness of many metallic, polymeric, and ceramic systems depends on the presence, at equilibrium, of various different phases in the material 组织

Condition of equilibrium S.J.T.U. Phase Transformation and Applications Two states are in equilibrium when no reversible work can be done by having the system change between those two states 2 6W,ev1->2=0 MA m Figure 4.1 Machine MA to trans- form mass m from state I to state 2. The temperatures in the two states must be equal .A consequence of the second law of thermodynamics .Otherwise... SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Condition of equilibrium • Two states are in equilibrium when no reversible work can be done by having the system change between those two states Wrev.1→2 = 0 • The temperatures in the two states must be equal •A consequence of the second law of thermodynamics •Otherwise…
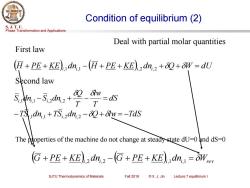
Condition of equilibrium(2) S.J.T.U. Phase Transformation and Applications Deal with partial molar quantities First law +PE+KE)a dn.-(+PE+KE).2dn2++W=du Second law 51-5,dm2+9 =ds T -TSdn.+TS,.dn.2-80+8w=-TdS The poperties of the machine do not change at steady state du-0and ds-0 G+PE+KE)dn2-G+PE+KE)dn.=OWc SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Condition of equilibrium (2) dS T lw T Q Si dni −Si dni + − = ,1 ,1 ,2 ,2 (H + PE + KE)i,1 d ni,1 −(H + PE + KE)i,2 d ni,2 +Q +W = dU Deal with partial molar quantities −TSi,1 dni,1 +TSi,2 dni,2 −Q +lw= −TdS First law Second law The properties of the machine do not change at steady state dU=0 and dS=0 ( ) ( ) G + PE + KE i,2 d ni,2 − G + PE + KE i,1 d ni,1 = Wrev
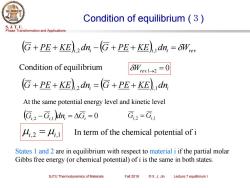
Condition of equilibrium(3) S.J.T.U. Phase Transformation and Applications G+PE+KE)dn,-G+PE+KE)dn,=OW Condition of equilibrium W,e1-→2=0 G+PE+KE)dn,=G+PE+KE)dn, At the same potential energy level and kinetic level (G,2-G,)dn,=AG,=0 G,2-G 4,2=4,1 In term of the chemical potential of i States 1 and 2 are in equilibrium with respect to material i if the partial molar Gibbs free energy (or chemical potential)of i is the same in both states. SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Condition of equilibrium (3) ( ) ( ) G + PE + KE i,2 d ni − G + PE + KE i,1 d ni = Wrev Condition of equilibrium Wrev.1→2 = 0 ( ) ( ) G + PE + KE i,2 d ni = G + PE + KE i,1 d ni (Gi,2 −Gi,1 )dni = Gi = 0 Gi,2 = Gi,1 At the same potential energy level and kinetic level i,2 = i,1 In term of the chemical potential of i States 1 and 2 are in equilibrium with respect to material i if the partial molar Gibbs free energy (or chemical potential) of i is the same in both states
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《材料热力学》教学资源_2018 lecture 6 property relation II.pdf
- 上海交通大学:《材料热力学》教学资源_2018 lecture 5 property relation.pdf
- 上海交通大学:《材料热力学》教学资源_2018 lecture 4 second law II.pdf
- 《力学改变生活》课程教学资源(文献资料)Observation of Gravitational Waves from a Binary Black Hole Merger.pdf
- 《力学改变生活》课程教学资源(文献资料)The First Sounds of Merging Black Holes.pdf
- 上海交通大学:《人体生物力学》课程教学资源(讲义)生物力学的发展前景.pdf
- 上海交通大学:《人体生物力学》课程教学资源(讲义)运动生物力学及组织损伤机理、计算机辅助手术.pdf
- 上海交通大学:《人体生物力学》课程教学资源(讲义)心血管生物力学及其在疾病诊疗方面的应用.pdf
- 上海交通大学:《人体生物力学》课程教学资源(讲义)人体生物力学概论(梁夫友).pdf
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_静平衡条件说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_运动学计算流程图.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_运动学上机_运动学计算流程图.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_双摆动力学逆问题说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_双摆动力学仿真说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_动力学附加题.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_动力学上机_静平衡条件说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_动力学上机_双摆动力学逆问题说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_动力学上机_双摆动力学仿真说明.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_Chapter 8_Modeling for Example2.doc
- 上海交通大学:《复杂系统动力学计算机辅助分析》课程教学资源_Chapter 8_Modeling for Example1.doc
- 上海交通大学:《材料热力学》教学资源_2018 lecture 8 chemical equilibrium.pdf
- 西安交通大学:《材料力学 Mechanics of Materials》课程教学资源(课件讲稿)第一部分 基本变形(共六章,主讲:殷民).pdf
- 西安交通大学:《材料力学 Mechanics of Materials》课程教学资源(课件讲稿)第二部分 组合变形(共三章,主讲:殷民).pdf
- 西安交通大学:《材料力学 Mechanics of Materials》课程教学资源(课件讲稿)第三部分 专题讨论(共四章,主讲:殷民).pdf
- 东莞理工学院:《材料力学 Mechanics of Materials》课程教学大纲.pdf
- 东南大学土木工程学院:《材料力学 Mechanics of Materials》课程教学资源(考核要求).pdf
- 上海交通大学材料科学与工程学院:《材料力学 Mechanics of Materials》课程教学大纲.pdf
- 西藏农牧学院:《结构力学》课程教学资源(发展简史,主讲:曹志翔).pdf
- 西藏农牧学院:《结构力学》课程教学资源(学科体系).pdf
- 西藏农牧学院:《结构力学》课程教学资源(课件教案与部分讲义)绪论、结构的几何组成、静定梁.pdf
- 西藏农牧学院:《结构力学》课程教学资源(教学单元教案,共十二章).pdf
- 西藏农牧学院:《结构力学》课程教学资源(求解器简介).pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(教学大纲,含思政元素).docx
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第一章 电磁现象的普遍规律.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第二章 静电场.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第三章 静磁场.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第四章 电磁波的传播.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第五章 电磁波的辐射.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(习题解答,打印版)第六章 狭义相对论.pdf
- 运城学院:《电动力学 Electrodynamics》课程教学资源(电子教案,打印版)绪论(负责人:郑伟).pdf
