北京化工大学:《物理化学》课程教学资源(双语习题课)Chemical equilibrium

Chemical equilibrium Tutorial Lecture PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
1 Chemical equilibrium Tutorial Lecture PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn f

1.For a chemical reaction, InK=a+b/T+C/T2.Derive the corresponding expressions to calculate△,Gm,△rlm,A,SOm and△,Cp,m PDF文件使用"pdfFactory Pro”试用版本创建mm,fineprint..com,cn
2 1. For a chemical reaction, lnK=a+b/T+c/T2 . Derive the corresponding expressions to calculate DrG0 m ,DrH0 m ,DrS 0 m and DrCp,m PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn

2.Assume that the following reaction is in equilibrium at 1000K: 3C(graphite)+2H2O(g)=CH4(g)+2CO(g) △,H0m(1000K=l82 kJmol-'.(a)What will be the effect on the equilibrium composition of raising the temperature at a total pressure of 1 bar?(b)What will be the effect of raising the pressure to 5 bar? OWhat will be the effect of adding nitrogen at a constant pressure of 1 bar? PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
3 2. Assume that the following reaction is in equilibrium at 1000K: 3C(graphite)+2H2O(g)=CH4 (g)+2CO(g) DrH0 m (1000K)=182kJmol-1. (a) What will be the effect on the equilibrium composition of raising the temperature at a total pressure of 1 bar?(b) What will be the effect of raising the pressure to 5 bar? ©What will be the effect of adding nitrogen at a constant pressure of 1 bar? PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

3.The following exothermic reaction is at equilibrium at 500K and 10 bar: CO(g)+2H2(g)=CH3OH(g) Assuming that the gases are ideal,what will happen to the amount of methanol at equilibrium when (a)the temperature is raised,(b)the pressure is increased,o an inert gas is pumped in at constant volume,(d)an inert gas is pumped in at constant pressure,and (e)hydrogen gas is added at constant pressure? PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint..com,cn
4 3.The following exothermic reaction is at equilibrium at 500K and 10 bar: CO(g)+2H2 (g)=CH3OH(g) Assuming that the gases are ideal, what will happen to the amount of methanol at equilibrium when (a) the temperature is raised,(b) the pressure is increased,© an inert gas is pumped in at constant volume,(d) an inert gas is pumped in at constant pressure, and (e) hydrogen gas is added at constant pressure? PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f
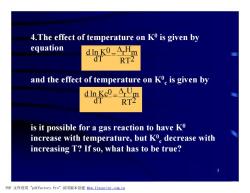
4.The effect of temperature on K0 is given by equation dink0=ArHm dT RT2 and the effect of temperature on Ko is given by dIn Kc0=ArUm dT RT2 is it possible for a gas reaction to have Ko increase with temperature,but K decrease with increasing T?If so,what has to be true? PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
5 4.The effect of temperature on K0 is given by equation and the effect of temperature on K0 c is given by is it possible for a gas reaction to have K0 increase with temperature, but K0 c decrease with increasing T? If so, what has to be true? RT2 r Hm dT d ln K0 D = RT2 r Um dT d ln Kc0 D = PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The First Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)双语教学大纲 Physical Chemistry.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)物理化学双语班《物理化学》教学大纲.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)理科实验班《物理化学》教学大纲.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)工科非化工类卓越工程师实验班《物理化学》教学大纲.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)工科化工类专业《物理化学》教学大纲.pdf
- 北京化工大学:《物理化学》课程教学资源(大纲教案)理科应用化学专业《物理化学》教学大纲.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第六章 氧化还原滴定法.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第七章 重量分析法和沉淀滴定法.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第五章 配位滴定法.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第四章 酸碱滴定法.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第三章 滴定分析概论.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第二章 误差及分析数据的统计处理.pdf
- 长沙理工大学:《分析化学》课程教学资源(课件讲稿)第一章 绪论(任课教师:李丹).pdf
- 长沙理工大学:《分析化学》课程教学资源(大纲教案)2020-2021学年第二学期教案.pdf
- 长沙理工大学:《分析化学》课程教学资源(大纲教案)2019-2020学年第二学期教案(任课教师:李丹).pdf
- 长沙理工大学:《分析化学》课程教学资源(作业复习)第八章 分光光度法(无答案).pdf
- 长沙理工大学:《分析化学》课程教学资源(作业复习)第七章 沉淀滴定法和重量分析法(无答案).pdf
- 长沙理工大学:《分析化学》课程教学资源(作业复习)第六章 氧化还原滴定法(无答案).pdf
- 长沙理工大学:《分析化学》课程教学资源(作业复习)第五章 配位滴定法(无答案).pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The thermodynamics of mixtures.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)Phase equilibrium.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第一章 气体英文习题及参考答案 gas problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第二章 热力学第一定律英文习题及参考答案 firstlaw problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第四章 多组分系统热力学英文习题及参考答案 mixture problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第三章 热力学第二定律英文习题及参考答案 secondlaw problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第七章 电化学英文习题及参考答案 electrochemistry problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第九章 化学反应动力学英文习题及参考答案 kinetics problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第八章 统计热力学初步英文习题及参考答案 staticcalthermodynamics problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第十章 表面化学英文习题及参考答案 surfaceandcolloid problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语实践教学)electrochemical determination of GHS.pdf
- 北京化工大学:《物理化学》课程教学资源(双语实践教学)kinetics the iodine clock reaction.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test four.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test one.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test three.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test two.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)air polution control.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Biosurfactants.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Explosion of battery.pdf
