北京化工大学:《物理化学》课程教学资源(双语读书报告)Biosurfactants

Biosurfactants B.Zhang h and Zhang wen Appled Chemistry 0501
Biosurfactants Biosurfactants By Zhang li and Zhang wen Applied Chemistry 0501

What is the biological surfacm Biological Surfactants are mic under certain conditions,culturein the metabolic process of secretion gi surfactant with the metabolite
Biological Surfactants are microbes under certain conditions, culture, in the metabolic process of secretion of surfactant with the metabolite What is the biological surfactants
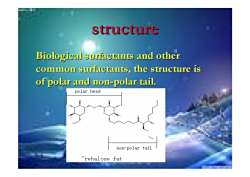
structure Biological surfactants and other common surfactants,the structure is of polar and non-polar tail. polar head non-polar tail Trehalose fat
structure structure Biological surfactants and other Biological surfactants and other common surfactants, the structure is common surfactants, the structure is of polar and non of polar and non-polar tail. polar tail

Biological surfaciani types General can be divided into 5 categories: Phospholipid T.thiooxidans Fatty Acids Hare Corynebacterium Lipopeptide and lipoprotein:Bacilus subilis Bacillus licheniformis Polymer LPS):B calcium Acinetobacter Glycolipid
General can be divided into 5 categories General can be divided into 5 categories: Phospholipid Phospholipid : T. thiooxidans : T. thiooxidans Fatty Acids Fatty Acids : Hare Corynebacterium : Hare Corynebacterium Lipopeptide and lipoprotein Lipopeptide and lipoprotein : Bacillus subtilis : Bacillus subtilis & Bacillus licheniformis Bacillus licheniformis Polymer (LPS) : Polymer (LPS) : B calcium Acinetobacter B calcium Acinetobacter Glycolipid Glycolipid

Glycolipid NAME THE GERM GROWS Trehalose fat Paraffin Arthrobacter Corynebacterium. Rhamnolipid Pseudomonas aeruginosa. Sophorolipid Xie Candida lipolytica. Glucose,fructose,sucrose fat Glabrata. Corynebacterium. Fiber two glycolipids Hung Ping Rhodococcus
Glycolipid Glycolipid NAME THE GERM GROWS NAME THE GERM GROWS Trehalose Trehalose fat Paraffin Arthrobacter. fat Paraffin Arthrobacter. & Corynebacterium. Corynebacterium. Rhamnolipid Rhamnolipid Pseudomonas aeruginosa. Pseudomonas aeruginosa. Sophorolipid Sophorolipid Xie Candida lipolytica. Xie Candida lipolytica. Glucose, fructose, sucrose fat Glucose, fructose, sucrose fat Glabrata. Glabrata. & Corynebacterium. Corynebacterium. Fiber two glycolipids Fiber two glycolipids Hung Ping Rhodococcus . Hung Ping Rhodococcus

Biological Surfactants Preparation 1 The microorganism ferments a method It is the cornmnonly used method,low cost, simple operation and can be used for almost all types of biosurfacants 2.Enzymatic conversion It is a method which makes use of the catalyst function of particular enzyme()to Synthesize biosurfacants directly
Biological Surfactants Biological Surfactants Preparation Preparation 1.The microorganism ferments a method The microorganism ferments a method It is the commonly used method, low cost, It is the commonly used method, low cost, simple operation and can be used for almost all simple operation and can be used for almost all types of biosurfacants types of biosurfacants 2.Enzymatic conversion Enzymatic conversion It is a method which makes use of the It is a method which makes use of the catalyst function of particular enzyme( catalyst function of particular enzyme(酶) to Synthesize biosurfacants directly. Synthesize biosurfacants directly
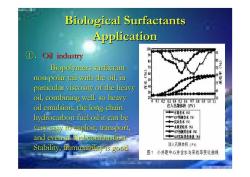
Biological Surfactants Application ① Oil industry 95 0 形 Biopolymers surfactant non-polar tail with the oil,in particular viscosity of the heavy 3 oil,combining well.so heavy 0020304050.6070.809101i oil emulsion,the long-chain 注入孔隙体积(PV) +一水驱含水() hydrocarbon fuel oil it can be “SP预测含水() ◆一实际含水() very easy to exploit,transport, ◆“水重采收率(角) ★一P预测采收率(传) and even as fuel combustion. ◆一S实际米收率值(修 Stability,flammability is good 注入孔隙体积(P) 图?小井距中心井含水与采收率变化曲线
Biological Surfactants Biological Surfactants Application Application ①.Oil industry Oil industry Biopolymers surfactant Biopolymers surfactant non -polar tail with the oil, in polar tail with the oil, in particular viscosity of the heavy particular viscosity of the heavy oil, combining well. so heavy oil, combining well. so heavy oil emulsion, the long oil emulsion, the long-chain hydrocarbon fuel oil it can be hydrocarbon fuel oil it can be very easy to exploit, transport, very easy to exploit, transport, and even as fuel combustion. and even as fuel combustion. Stability, flammability is good Stability, flammability is good

②.Medicinal Lecithin now face world class health products can be seen everywhere For instance phospholipids are also biological material surfactant A,in the role of the human body is to accelerate the metabolism of fat substances
②.Medicinal Medicinal Lecithin now face world class health Lecithin now face world class health products can be seen everywhere products can be seen everywhere For instance phospholipids are also For instance phospholipids are also biological material surfactant A, in the biological material surfactant A, in the role of the human body is to accelerate role of the human body is to accelerate the metabolism of fat substances the metabolism of fat substances

degradation of toxic substances Biological Surfactants especially Surfactin good material to the adsorption of heavy metal ions and hydroxide formed chelates C-CH(CH2)-CH-CH2-CO-GLU-LEU-LEU Amino acids HEU一⊥EU-ASP surfactin
③degradation of toxic substances degradation of toxic substances Biological Surfactants especially Surfactin good Biological Surfactants especially Surfactin good material to the adsorption of heavy metal ions and material to the adsorption of heavy metal ions and hydroxide formed chelates hydroxide formed chelates Amino acids
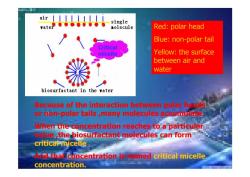
air single 量olecule Red:polar head Blue:non-polar tail Critical micelle Yellow:the surface between air and water biosurfactant in the vater Because of the interaction between polar hgls or hon-polar tails ,many molecules accumulate When the concentration reaches to a particular e the biosurfactant molecules can form critical micelle dthali concentration is omed critical micelle concentration
Red: polar head Blue: non-polar tail Yellow: the surface between air and water Critical micelle Because of the interaction between polar heads or non-polar tails ,many molecules accumulate. When the concentration reaches to a particular value ,the biosurfactant molecules can form critical micelle. And that concentration is named critical micelle concentration
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)air polution control.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test two.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test three.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test one.pdf
- 北京化工大学:《物理化学》课程教学资源(双语模拟试题)test four.pdf
- 北京化工大学:《物理化学》课程教学资源(双语实践教学)kinetics the iodine clock reaction.pdf
- 北京化工大学:《物理化学》课程教学资源(双语实践教学)electrochemical determination of GHS.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第十章 表面化学英文习题及参考答案 surfaceandcolloid problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第八章 统计热力学初步英文习题及参考答案 staticcalthermodynamics problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第九章 化学反应动力学英文习题及参考答案 kinetics problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第七章 电化学英文习题及参考答案 electrochemistry problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第三章 热力学第二定律英文习题及参考答案 secondlaw problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第四章 多组分系统热力学英文习题及参考答案 mixture problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第二章 热力学第一定律英文习题及参考答案 firstlaw problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语练习题)第一章 气体英文习题及参考答案 gas problems.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)Phase equilibrium.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The thermodynamics of mixtures.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)Chemical equilibrium.pdf
- 北京化工大学:《物理化学》课程教学资源(双语习题课)The First Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Explosion of battery.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Rain Making.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Lithium Battery.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)lithium ion battery.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Nano lantern.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)nanotechnology.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)Surface Tension.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)surfactant in cosmetic.pdf
- 北京化工大学:《物理化学》课程教学资源(双语读书报告)What is Colloid.pdf
- 《物理化学》课程教学资源(数值计算)01 范德华气体(Van der Waals状态方程式).pdf
- 《物理化学》课程教学资源(数值计算)02 化学反应焓熵吉布斯函数与反应温度的关系.pdf
- 《物理化学》课程教学资源(数值计算)03 最高火焰温度计算.pdf
- 《物理化学》课程教学资源(数值计算)04 克劳修斯-克拉佩龙方程.pdf
- 《物理化学》课程教学资源(数值计算)05 实际气体逸度计算.pdf
- 《物理化学》课程教学资源(数值计算)06 压力对平衡转化率的影响.pdf
- 《物理化学》课程教学资源(数值计算)07 电动势法测离子平均活度系数.pdf
- 《物理化学》课程教学资源(数值计算)08 半衰期法确定动力学方程.pdf
- 《物理化学》课程教学资源(数值计算)09 微分法确定动力学方程.pdf
- 《物理化学》课程教学资源(数值计算)10 溶液吸附量测量.pdf
- 北京化工大学:《物理化学》课程教学资源(研讨报告)金属的电化学腐蚀与防腐.pdf
