北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 14 p-block elements(Ⅱ)

Chapter 14 The p-block elements(II) X 14.1 The elements of nitrogen family 14.2 The elements of oxygen family
Chapter 14 The p-block elements( Ⅱ ) §14.2 The elements of oxygen family §14.1 The elements of nitrogen family

14.1 The elements of nitrogen family -14.1.1 Outline on nitrogen family 14.1.2 The elemental substances of nitrogen family 14.1.3 The compounds of nitrogen 14.1.4 The compounds of phosphorus -14.1.5 The compounds of As,Sb and Bi
14.1.5 The compounds of As, Sb and Bi 14.1.4 The compounds of phosphorus 14.1.3 The compounds of nitrogen 14.1.2 The elemental substances of nitrogen family 14.1.1 Outline on nitrogen family §14.1 The elements of nitrogen family

14.1.1 Outline on nitrogen family The elements of nitrogen family(VA):N,P,As,Sb,Bi Valence electron configuration:ns2np3 N P As Sb Bi 氧化值 +5 +5 +5 +5 (+5) +3 +3 +3 +3 -3 -3 -3 (-3) 最大配 4 位数 6 6 6 6 M,O; 酸性 酸性 两性 两性 碱性 MH; NH:PH3 AsH3 SbH3 BiH 碱性减弱,稳定性下降
14.1.1 Outline on nitrogen family The elements of nitro gen famil y(VA) :N, P, As, Sb, Bi Valence electron configuration : n s 2 n p 3 N P As Sb Bi +5 +5 +5 (+5) +3 +3 +3 +3 氧化值 +5 | -3 -3 -3 (-3) 最大配 位数 4 6 6 66 M 2 O 3 酸性 酸性 两性 两性 碱性 氨 膦 胂 SbH 3 BiH 3 MH 3 碱性减弱,稳定性下降 NH 3 PH 3 AsH 3 SbH 3 BiH 3

14.1.2 The elemental substances of nitrogen family N,is colorless and odorless gas.N2 is chemically inert.It will react directly with many metals(such as Li,Ca,Mg)when heated to give ionic nitrides. Nitrogen is also easily converted to a liquid (b.p, 195.8'C)and can dissolve in water slightly
N2 is colorless and odorless gas. N2 is chemically inert. It will react directly with many metals (such as Li, Ca, Mg) when heated to give ionic nitrides. Nitrogen is also easily converted to a liquid (b.p, - 195.8°C) and can dissolve in water slightly. 14.1.2 The elemental substances of nitrogen family

The allotropes of phosphorus 黑磷←高温高压 一白磷 隔绝空气400℃→红磷 net framework, P:chemically relatively stable, conductive, active,autoignite in combust at insoluble in org air,soluble in T>400°C,insoluble solv. nonplolar solv. in org.solv. Self-ignite in air白 combustion of P 白磷红磷 磷空气中自燃 磷的燃烧
白磷 红磷 The allotropes of phosphorus 黑磷 ←⎯ ⎯ 高温高压 ⎯⎯⎯ 白磷 ⎯隔绝空气 ⎯ → ⎯⎯⎯400⎯°⎯C 红磷 P4 : chemically active, autoignite in air, soluble in nonplolar solv. relatively stable, combust at T>400°C, insoluble in org. solv. combustion of P 磷的燃烧 Self-ignite in air白 磷空气中自燃 net framework, conductive, insoluble in org. solv
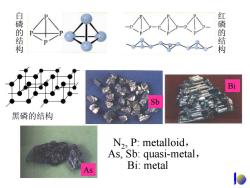
白磷的结构 红磷的结构 对 Bi Sb 黑磷的结构 N2,P:metalloid, As,Sb:quasi-metal, Bi:metal
As Sb Bi N2, P: metalloid, As, Sb: quasi-metal, Bi: metal 白磷的结构 红磷的结构 黑磷的结构

14.1.3 The compounds of nitrogen 1.Hydride of nitrogen ◆ammonia(NH3) molecular N:sp3 hybridization,triangular structure: pyramid molecular structure N 100.8pm 107.30
14.1.3 The compounds of nitrogen molecular structure: N :sp 3 hybridization, triangular pyramid molecular structure 1. Hydride of nitrogen N H 100.8pm 107.3 o .. H H ammonia (NH 3 )

Preparation: In laboratory: 2NHCI+Ca(OH),>CaCl,+2H2O+2NH3(g) 雨林木风1 In industry process: N2+3H2450-500C30MPae→2NH, Chemical properties: 1 Soluble in water forming monoprotic weak base NH+H,ONH·H,O-NH4+OH 2 Strong reducing ability 4NH3 +302(pure)->2N2+6H2O 4NH,+50,(ain0→4N0+61,0
In industry process: 2NH Cl Ca(OH) CaCl 2H O 2NH (g) 4 + 2 ⎯⎯→ 2 + 2 + 3 N 2 3H 2 2NH 3 ⎯ 450 ⎯ → ⎯~500 ⎯⎯C 30MPa ⎯⎯⎯⎯ Fe + ° Preparation : In laboratory : Chemical properties : ① Soluble in water forming monoprotic weak base ② Strong reducing ability _ NH3 + H 2 O NH3 ⋅H 2 O NH4 +OH + 4NH 5O (air) 4NO 6H O 4NH 3O (pure) 2N 6H O 2 Pt 3 2 3 2 2 2 + ⎯⎯→ + + → + 800 o c 雨林木风1

3 coordination reaction Ht+NH3→NH4 Ag+2NH3→[AgNH3)2]H 4Substitution reaction 2NH,+2Na- 70c→2NaNH2+H2 催化 ◆NH,-NH,联氨(肼),NH氏亚氨基,N<氮化物 hydrazine ◆NH,OH羟氦,Ammonium hydroxide
④ Substitution reaction + + + + + → + → Ag 2NH [Ag(NH ) ] H NH NH 3 3 2 3 4 2 2 570 C 2NH3 + 2Na ⎯⎯ →⎯ 2NaNH + H ° 催化 ③ coordination reaction NH2-NH2联氨(肼),NH 亚氨基,N 氮化物 hydrazine NH2OH 羟氨, Ammonium hydroxide
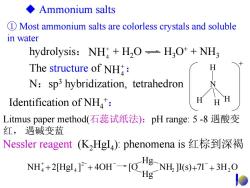
◆Ammonium salts D Most ammonium salts are colorless crystals and soluble in water hydrolysis:NH H2O-H3O++NH3 The structure of NH: N:sp3 hybridization,tetrahedron Identification of NH: Litmus paper method(石蕊试纸法):pH range:5-8遇酸变 红,遇碱变蓝 Nessler reagent(KHgI4):phenomena is红棕到深褐 NH4+2[Hgl4]2+4OH→[O g-NH.]I(s)+7+3H.O Hg
① Most ammonium salts are colorless crystals and soluble in water H N H H H + Ammonium salts Litmus paper method(石蕊试纸法):pH range: 5 -8 遇酸变 红, 遇碱变蓝 + NH4 The structure of : Nessler reagent (K2HgI4): phenomena is 红棕到深褐 N:sp3 hybridization, tetrahedron Identification of NH4+: NH ]I(s) 7I 3H O Hg Hg NH 2[HgI ] 4OH [O 2 2 2 4 + 4 + + + + − − − hydrolysis: + H2O H3O+ + NH3 + NH4
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 17 The d-block elements(Ⅱ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 09 Molecular Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 07 Redox Reactions and Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 13 The p-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 08 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 06 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 12 The s-Block Elements.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 11 Coordination Compound Structures.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 10 Solid Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 04 Chemical equilibria, entropy and Gibbs function.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 05 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 03 Chemical kinetics.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 02 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 01 前言 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六-十七综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十七章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十五章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十三-十四章综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十四章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 15 p-block elements(Ⅲ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 16 The d-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 2 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 5 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 6 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 7 Redox Reactions and the Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 8 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 1 Preface.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 2 Thermochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 5 Acid-Base Equilibrium.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 6 Precipitation-Solubility Equilibria.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 4 Chemical equilibria, entropy and Gibbs function.ppt
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 4 Chemical equilibria, entropy and Gibbs function.pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 10 Solid Structure.pptx
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 11 Coordination Compound Structures.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 12 The s-Block Elements.pptx
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 13 The p-block elements(Ⅰ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 14 Chapter 14 The p-block elements(Ⅱ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 15 The p-block elements(Ⅲ).ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 16 The d-block elements(Ⅰ).ppt
