北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 11 Coordination Compound Structures

Chap 11 Coordination Compound Structures X 11.1 Configurations and Magnetism of Coordination Compounds X $11.2 Chemical Bonding Theory in Complexes
Chap 11 Coordination Compound Structures §11.2 Chemical Bonding Theory in Complexes §11.1 Configurations and Magnetism of Coordination Compounds

11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1.configurations 2.isomers 11.1.2 Magnetism of Coordination Compounds
11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1. configurations 2. isomers 11.1.2 Magnetism of Coordination Compounds
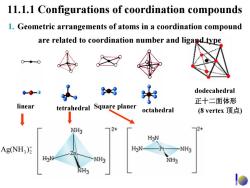
11.1.1 Configurations of coordination compounds 1.Geometric arrangements of atoms in a coordination compound are related to coordination number and ligand type 00 00-0 88 dodecahedral 正十二面体形 linear tetrahedral Square planer octahedral (8 vertex顶点) NH3 2+ 2+ AgNH3)克 H3N- NH3 H-N NH3 NH3 o
11.1.1 Configurations of coordination compounds 1. Geometric arrangements of atoms in a coordination compound are related to coordination number and ligand type linear Square planer tetrahedral octahedral dodecahedral 正十二面体形 (8 vertex 顶点 ) + 23 )Ag(NH
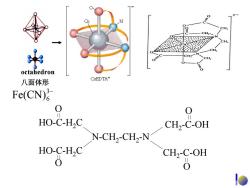
octahedron 八面体形 CoEDTA- Fe(CN) 0 0 HO-C-H2C CH,-C-OH HO-C-H2C N.CH.-CIN CH2-C-OH 0 0
O HO-C-H2C O HO-C-H2C N-CH2-CH2-N O CH2-C-OH O CH2-C-OH 乙二胺四乙酸 octahedron 八面体形 3− Fe(CN)6
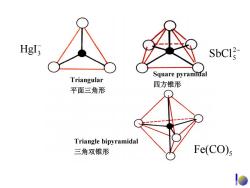
Hgl, 不N SbCI Square pyramidal Triangular 四方锥形 平面三角形 Triangle bipyramidal 三角双锥形 Fe(CO)s
Triangular 平面三角形 Square pyramidal 四方锥形 Triangle bipyramidal 三角双锥形 − HgI3 2− SbCl5 Fe(CO)5

83 Triangular Square pyramidal Square planer linear tetragonal octahedral Rules 1.In chemistry,a coordination complex or metal complex,is a structure consisting of Triangle bipyramidal a central atom or ion (usually metallic), bonded to a surrounding array of molecules or anions (ligands,complexing agents). 2.Ligands tend to stay away to have low energy and stability
linear Square planer octahedral Triangular Square pyramidal Triangle bipyramidal tetragonal • 1.In chemistry, a coordination complex or metal complex, is a structure consisting of a central atom or ion (usually metallic), bonded to a surrounding array of molecules or anions (ligands, complexing agents). • 2.Ligands tend to stay away to have low energy and stability Rules

2.stereoisomer of coordination compounds isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement. Structural isomers Stereoisomer NH3 24 NH3 H N 1 的H3 NH3 NH3 (a) (b)
◆ isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement。 2. stereoisomer of coordination compounds Structural isomers Stereoisomer
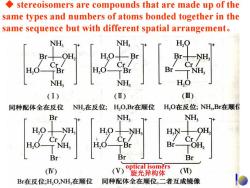
stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement. NH NH, H,O Br十 Br十 Cr Cr/ Cr/ H,C H,0 TBr Br-NH, NH, NH H,O (I) (Ⅱ) (Ⅲ) 同种配体全在反位 NH,在反位; H,O,Br在顺位 H,O在反位;NH,Br在顺 Br NH, NH, NH H, OH, Cr/ Cr/ Cr/ H,O- r NH, H,O Br- TOH, Br Br Br optical isomers (V) (V) 旋光异构体 (I) Br在反位;H,O,NH,在顺位 同种配体全在顺位,二者互成镜像
◆ stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement。 optical isomers 旋光异构体
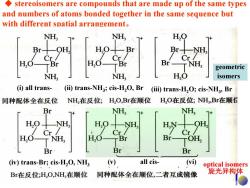
stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement. NH, NH, H,Q Br- H0十 Br Br十 NH Cr Cr H,O TBr Br- T NH geometric NH, NH H,O isomers (i)all trans- (ii)trans-NH3;cis-H2O,Br (iii)trans-H2O;cis-NH3,Br 同种配体全在反位 NH,在反位;H,O,Br在顺位 H,O在反位;NH,Br在顺付 Br NH; NH, H.O NH, HN- OH, Cr/ Cr Cr H,O-NH, H,0 TBr Br- TOH Br Br Br (iv)trans-Br;cis-H2O,NH3 () all cis- (vi) optical isomers Br在反位;H,O,NH,在顺位 同种配体全在顺位,二者互成镜像 旋光异构体
◆ stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement。 optical isomers 旋光异构体 geometric isomers (i) all trans- (ii) trans-NH3; cis-H2O, Br (iii) trans-H2O; cis-NH3, Br (iv) trans-Br; cis-H2O, NH3 (v) all cis- (vi)
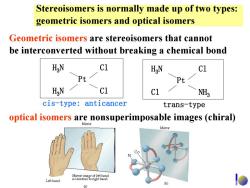
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond HaN C1 H N c1 Pt Pt HaN c1 NH cis-type:anticancer trans-type optical isomers are nonsuperimposable images (chiral) Mirror Left hand 01
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond optical isomers are nonsuperimposable images (chiral) H 3 N H 3 N Pt Cl Cl H 3 N Cl Pt Cl NH 3 cis-type: anticancer trans-type
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 10 Solid Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 04 Chemical equilibria, entropy and Gibbs function.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 05 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 03 Chemical kinetics.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 02 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 01 前言 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六-十七综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十七章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十五章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十三-十四章综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十四章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十二章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十三章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十一章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第八章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第九章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第七章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第四章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 12 The s-Block Elements.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 06 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 08 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 13 The p-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 07 Redox Reactions and Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 09 Molecular Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 17 The d-block elements(Ⅱ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 14 p-block elements(Ⅱ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 15 p-block elements(Ⅲ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 16 The d-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 2 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 5 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 6 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 7 Redox Reactions and the Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 8 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 1 Preface.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 2 Thermochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 5 Acid-Base Equilibrium.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 6 Precipitation-Solubility Equilibria.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 4 Chemical equilibria, entropy and Gibbs function.ppt
