北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 12 The s-Block Elements

Chapter 12 The s-Block Elements 12.1 The general properties of the s-block elements X 12.2 The elementary substances קl2.3 The properties of the alkali and alkaline earth compounds X$12.4 The particularity of Li and Be The diagonal relationship
§12.1 The general properties of the s-block elements §12.4 The particularity of Li and Be The diagonal relationship §12.3 The properties of the alkali and alkaline earth compounds §12.2 The elementary substances Chapter 12 The s-Block Elements

§12.1 The general properties of the s-block elements The alkali metals(IA )ns Li,Na,K,Rb,Cs,Fr The alkaline metals (IIA )ns2 Be,Mg,Ca,Sr,Ba,Ra They are all active metals e
§12.1 The general properties of the s-block elements The alkali metals (IA ):ns1 Li, Na, K, Rb, Cs, Fr The alkaline metals (IIA ):ns2 Be, Mg, Ca, Sr, Ba, Ra They are all active metals

IA IIA electronegativity Decreasing g ionization energy and Increasing metallicity Increasing atomic Li Be Na Mg K Ca Rb Sr radius Cs Ba reductibility Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity
electronegativity Decreasing ionization energy and Increasing metallicity and reductibility Increasing atomic radius Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity IA IIA Li Be Na Mg K Ca Rb Sr Cs Ba

General properties of S block elemental substancs 1.React with H2 to produce ionic compounds M+1H-1, M+2H-12 (except for Be); 2.React with O,to form oxide,peroxide,superoxide; 3.React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4.Dissolve into liquid NH3 to form blue reducing solution except for Be Note:they show different activities
1. React with H2 to produce ionic compounds M+1H-1、 M+2H-12 (except for Be); 2. React with O2 to form oxide, peroxide, superoxide; 3. React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4. Dissolve into liquid NH3 to form blue reducing solution except for Be General properties of S block elemental substancs Note: they show different activities

S 12.2 The elementary substances 12.2.1 The properties of the elementary substances 1.Physical properties ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; *Good conductor of heat and electricity Na K
§12.2.1 The properties of the elementary substances Na Li K 1.Physical properties §12.2 The elementary substances ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; ☆Good conductor of heat and electricity

Rb Cs 181 Low melting point Be Mg a Sr Ba Li Na KRb Cs Fr
Be Mg Ca Sr Ba Rb Cs Low melting point Li Na K Rb Cs Fr

2.Chemical properties: ·React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. The elementary substances form the corresponding oxides when they burn in air: LiO Na202 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 Na,O2 Li,C Pale yellow K Pale yellow Magnesium burning in air
The elementary substances form the corresponding oxides when they burn in air: Li2O Na2O2 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 • React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. 2.Chemical properties: Magnesium burning in air Li2O Na2O2 KO2 Pale yellow Pale yellow

.React with water 2M+2H,O-2MOH+H2(g) Li Na K Bromothymol blue indicator 溴百里酚兰指示剂 Ca
•React with water Li Na K Ca 2M + 2H 2O → 2MOH + H 2(g) Bromothymol blue indicator 溴百里酚兰 指示剂
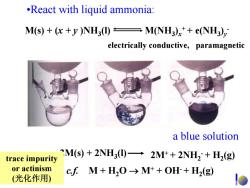
.React with liquid ammonia: M(s)+(x+y)NH3(I)M(NH3)x++e(NH3), electrically conductive,paramagnetic a blue solution trace impurity CM(s)+2NH3(I)-2M++2NH2+H2(g) or actinism cfM+H2O→Mt+OH+H2(g) (光化作用)
•React with liquid ammonia: a blue solution 2M(s) + 2NH 3(l) 2M+ + 2NH 2 - + H 2(g) c.f. M + H 2O → M + + OH - + H 2(g) M(s) + (x + y )NH 3(l) M(NH 3 ) x + + e(NH 3 )y - electrically conductive, paramagnetic trace impurity or actinism (光化作用 )
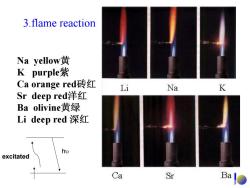
3.flame reaction Na yellow黄 K purple紫 Ca orange red砖红 Li Na K Sr deep red洋红 Ba olivine黄绿 Li deep red深红 excitated Ca Sr Ba
3.flame reaction Na yellow 黄 K purple 紫 Ca orange red砖红 Sr deep red洋红 Ba olivine黄绿 Li deep red 深红 excitated h υ
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 11 Coordination Compound Structures.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 10 Solid Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 04 Chemical equilibria, entropy and Gibbs function.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 05 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 03 Chemical kinetics.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 02 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 01 前言 Preface(负责人:周云山).pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六-十七综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十六章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十七章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十五章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十三-十四章综合自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十四章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十二章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十三章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第十一章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第八章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第九章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(试卷习题)第七章自我练习题及答案.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 06 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 08 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 13 The p-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 07 Redox Reactions and Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 09 Molecular Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 17 The d-block elements(Ⅱ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 14 p-block elements(Ⅱ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 15 p-block elements(Ⅲ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2011)Chapter 16 The d-block elements(Ⅰ).pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 2 Thermochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 5 Acid-Base Equilibrium.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 6 Precipitation-Solubility Equilibria.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 7 Redox Reactions and the Base of Electrochemistry.pdf
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 8 Atomic Structure.pdf
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 1 Preface.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 2 Thermochemistry.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 5 Acid-Base Equilibrium.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 6 Precipitation-Solubility Equilibria.ppt
- 北京化工大学:《无机化学》课程电子教案(PPT课件,2013)Chapter 4 Chemical equilibria, entropy and Gibbs function.ppt
- 北京化工大学:《无机化学》课程电子教案(教学课件,2012)Chapter 4 Chemical equilibria, entropy and Gibbs function.pdf
