同济大学:《大学物理》课程教学资源(电子教案,英文版)原子物理1

Atomic Physics RESERVE BANKOP NEW ZEALAND 00g AD273409 RITHERFORD NELSON THIS NOTE JS LEGAL TENDER EOR ONE HUNDRED DOLLARS
Atomic Physics

Agenda today 1.Spectrum of hydrogen atoms 2.Bohr's model for hydrogen atom 3.Schodiger's equation for hydrogen atom 4.The wave function of hydrogen atom
Agenda today 1. Spectrum of hydrogen atoms 2. Bohr’s model for hydrogen atom 3. Schodiger’s equation for hydrogen atom 4. The wave function of hydrogen atom
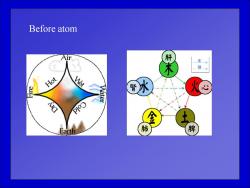
Before atom 肝 生 Hot Met 腎 Earth 肺 脾
Before atom
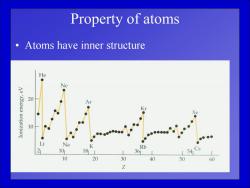
Property of atoms Atoms have inner structure He Ne Aa'A3aua uonezruoI 20 Ar 10 ● Li Na K Rb 21 101 18 361 L54 10 20 30 40 50 60 Z
Property of atoms • Atoms have inner structure

Atoms emit and absorb light hf-Enigh-Elow
Atoms emit and absorb light hf=Ehigh-Elow

Atoms have angular momentum and magnetism
Atoms have angular momentum and magnetism

Atomic spectra(原子光谱) Emission spectrum(发射光谱) H,H。 7000A 6563A 6000A 5000A4861A4340A4000A Absorption spectrum(吸收光谱)
Atomic spectra(原子光谱) Emission spectrum(发射光谱) 7000A 6563A 6000A 5000A 4861A 4340A 4000A H H H H Absorption spectrum(吸收光谱)

Balmer series(巴尔末系) Wave number 1 节= 2 v=R Johann Jakob Balmer (1825- 1898)and spectra of atomic the lower spectrum shows n=3,4, Baimer lines in the ultraviolet Rg=1.096776×10m Rydberg constant
Balmer series(巴尔末系) n 3,4, Wave number 7 1 1.096776 10 RH m Rydberg constant

Lyman series -非 n=2,3, Paschen series n=4,5, Brackett series V-Ru n=5,6,…
Lyman series n 2,3, Paschen series n 4,5, Brackett series n 5,6,
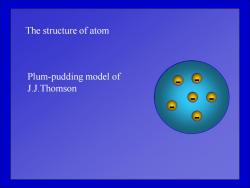
The structure of atom Plum-pudding model of J.J.Thomson
The structure of atom - - - - - - Plum-pudding model of J.J.Thomson
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十章 气体动力论.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十一章 热力学(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第六章 刚体的转动(下)..pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第六章 刚体的转动(上)..pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第八章 振动.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(中).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第七章 引力.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二章 质点的运动.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第三章 牛顿定律.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第一章 测量(负责人:王治国).pdf
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-13 超导电性.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-12 半导体.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-11 激光.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-08 量子力学简介.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-07 不确定关系.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-06 德布罗意波、实物粒子的二象性.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-05 夫兰克-赫芝实验.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-04 玻尔理论.ppt
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)原子物理2.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十一章 相对论.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十三章 固体物理.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十章 光学(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十章 光学(中).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十一章 热力学(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十七章 交流电路.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十三章 高斯定律.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十九章 电磁波.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十二章 电场与电势(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十二章 电场与电势(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十五章 磁场(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十五章 磁场(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十八章 物质的磁性.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十六章 电磁感应.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十四章 直流电路.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子1.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子2.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子3.pdf
- 安徽科技学院:《大学物理》课程教学资源(PPT课件)电学——第八章 静电场中的导体和电介质(Conductors and Dielectrics in Electrostatic Field).ppt
